Fig. 4.
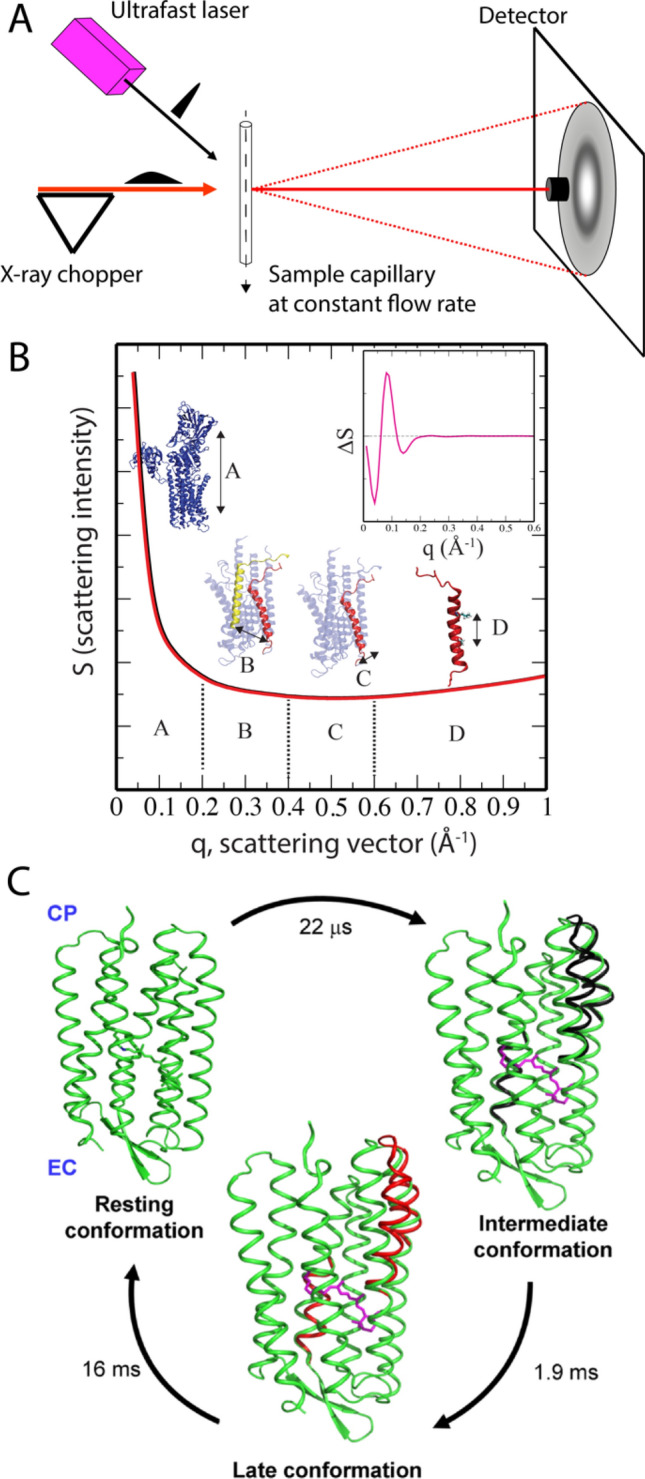
Schematic of the TR-XSS experimental design and resolved membrane protein dynamics. a The pump laser pulse arrives at the sample in the capillary (either static or at a continuous flow rate) before the onset of the X-ray probe pulse, which yields concentric rings on the detector. Subtracting non-activated images from the laser-activated images results in difference images that contain the time-resolved structural data. b Conventional X-ray scattering profiles from two P-type ATPase protein structures (black and red lines), where q = 4π sin(θ)/λ = 4π/2d, where 1/d is the resolution in X-ray crystallography. E.g. q = 0.2 Å−1 corresponds to distances of approximately 30 Å, and q = 1.0 Å−1 to approximately 6 Å. An example of a difference TR-XSS spectrum is shown in the inset. c Bacteriorhodopsin TR-XSS data identified structures and kinetics of two transient intermediates at 22 μs and 1.9 ms with a subsequent relaxation back to the ground state in 16 ms. The structural changes were about twice those observed in crystal structures and revised the bacteriorhodopsin transport mechanism. Adapted from Andersson et al. (2009)
