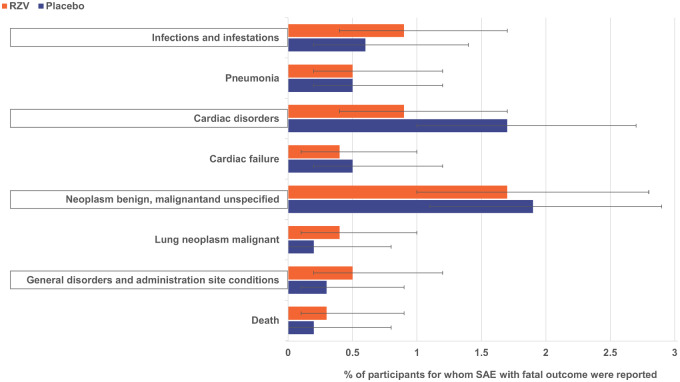
Fig. 3 SAEs with fatal outcome in participants with pre-existing pIMDs reported during the entire study period (TVC)
SAEs by MedDRA SOC (framed) and PT are presented here. Per PT, only SAEs reported by ≥0.3% of RZV recipients are presented here. Error bars represent 95% CI. MedDRA: Medical Dictionary for Regulatory Activities; pIMD: potential immune-mediated disease; Placebo: participants receiving placebo; PT: preferred term; RZV: participants receiving the adjuvanted recombinant zoster vaccine; SAE: serious adverse event; SOC: system organ class; TVC: total vaccinated cohort.