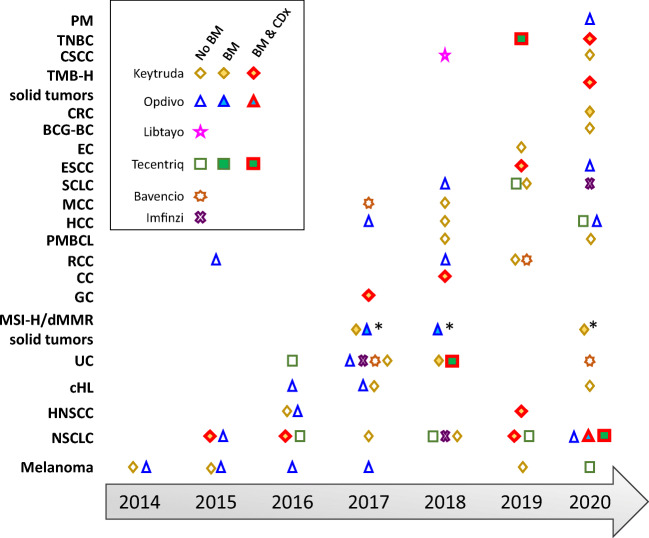
Fig. 1.
FDA approvals of PD-1/PD-L1 mAbs. As of December 2020, six anti-PD-1/PD-L1 mAbs have been approved with supplemental indications across 19 cancer types and two tissue-agnostic conditions. Shown are the approvals for each cancer indication, for Keytruda (pembrolizumab), Opdivo (nivolumab), Libtayo (cemiplimab), Tecentriq (atezolizumab), Bavencio (avelumab), and Imfinzi (durvalumab). Multiple approvals for a cancer indication within the same year are shown with only one symbol. The open symbols represent approvals without a biomarker (no BM). The full symbols represent approvals that incorporate a biomarker with an associated threshold for each indication (BM), which was measured using either a central laboratory test or complementary diagnostic that was not approved as a CDx for the drug. Symbols with a red outline represent approvals in which a companion diagnostic is indicated for biomarker measurement (BM + CDx). *: approval for MSI-H/dMMR colorectal cancer. PM, pleural mesothelioma; TNBC, triple-negative breast cancer; CSCC, cutaneous squamous cell carcinoma; TMB-H, tumor mutation burden high; CRC, colorectal cancer; BCG-BC, Bacillus Calmette-Guérin bladder cancer; EC, endometrial carcinoma; ESCC, esophageal squamous cell carcinoma; SCLC, small cell lung cancer; RCC, renal cell carcinoma; MCC, Merkel cell carcinoma; HCC, hepatocellular carcinoma; PMBCL, primary mediastinal large B cell lymphoma; CC, cervical cancer; GC, gastric cancer; MSI-H, microsatellite instability high; dMMR, mismatch repair-deficient; UC, urothelial carcinoma; cHL, classical Hodgkin’s lymphoma; HNSCC, head and neck squamous cell carcinoma; NSCLC, non-small cell lung cancer. Information on approvals and supplemental approvals was gathered from Drugs@FDA