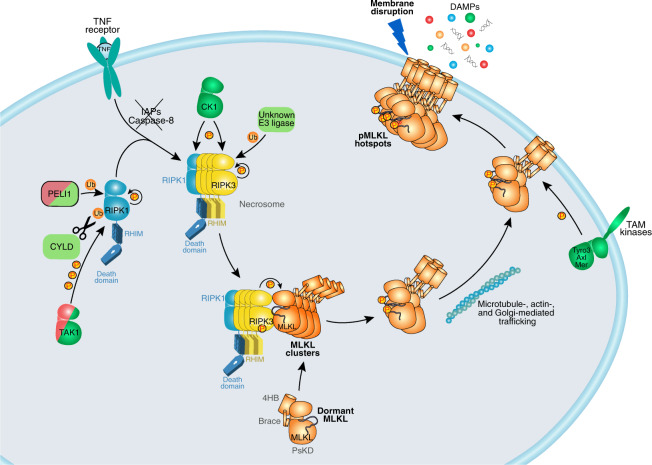
Fig. 2. Post-translational modifications positively regulate necroptosis.
RIPK1 is positively regulated by K63-linked ubiquitination (PELI1), removal of M1-linked ubiquitin chains (CYLD), and autophosphorylation. RIPK3 autophosphorylation is required for its interaction with, and phosphorylation of, MLKL. This is promoted by RIPK3 ubiquitination at K5, as well as CK1-mediated phosphorylation in some contexts. TAM kinase-mediated phosphorylation was proposed to promote MLKL clustering at plasma membrane into hotspots.