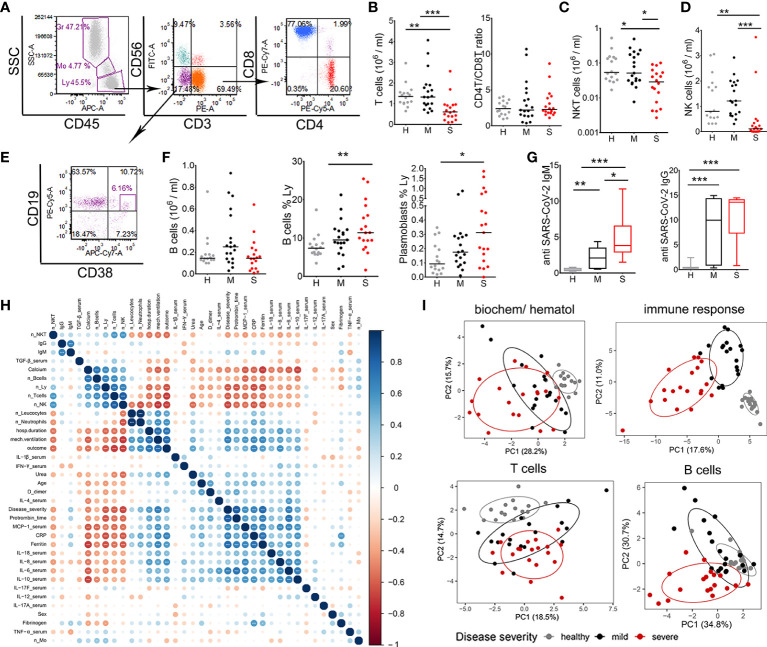
Figure 1.
Lymphocytes characterization and correlations with clinical parameters of coronavirus disease (COVID)-19. (A) Representative gating of lymphocyte populations is shown. Doublets were excluded by FSc-A/FSc-H (not shown) and lymphocytes (Ly) (CD45hiSSclow) were gated to identify NKT (CD3+CD56+), NK (CD3-CD56+), and T (CD3+CD56-) cells. T cells were gated to identify CD4 and CD8 T cell subsets. (B) The number of T cells/ml was calculated from the percentage of cells and the number of leukocytes for each donor in the groups of healthy donors (H, n=16), mild (M, n=19) and severe (S, n=19) patients. CD4/CD8 T cell ratio was calculated from the percentage of CD4+ and CD8+ T cells in lymphocytes region. The numbers of NKT (C) and NK cells (D) were calculated as in (B). (E) B cells (CD19+) and plasmablasts (CD19+CD38hi) were gated from CD3-CD56- region as indicated. (F) The number of B cells/ml, the relative percentage of B cells and plasmablasts in lymphocytes region are shown. (G) The levels of SARS-CoV-2-specific IgM and IgG antibodies in sera of patients (mild n=21, severe n=20) and healthy donors (n=16) are shown as Tukey box-whiskers. (B, D, F, G) *p < 0.05, **p < 0.01, ***p < 0.005 as indicated (Kruskal-Wallis test with Dunn’s post-test). (H) Spearman’s correlation matrix and hierarchical clustering of 36 common features in 19 mild and 19 severe COVID-19 patients. The color and size of the circles represent the correlation coefficient. Asterisks indicate significance levels for each comparison, at FDR below 0.05 (*), 0.01 (**), and 0.001 (***). n_, number; Mo- monocytes. (I) Principal component analysis (PCA) of hematological and biochemical (n=17), immune response (n=144), T cells (n=35), B cells and antibody data (n=11) are shown with 95% confidence ellipse for healthy donors, mild, and severe COVID-19 patients.