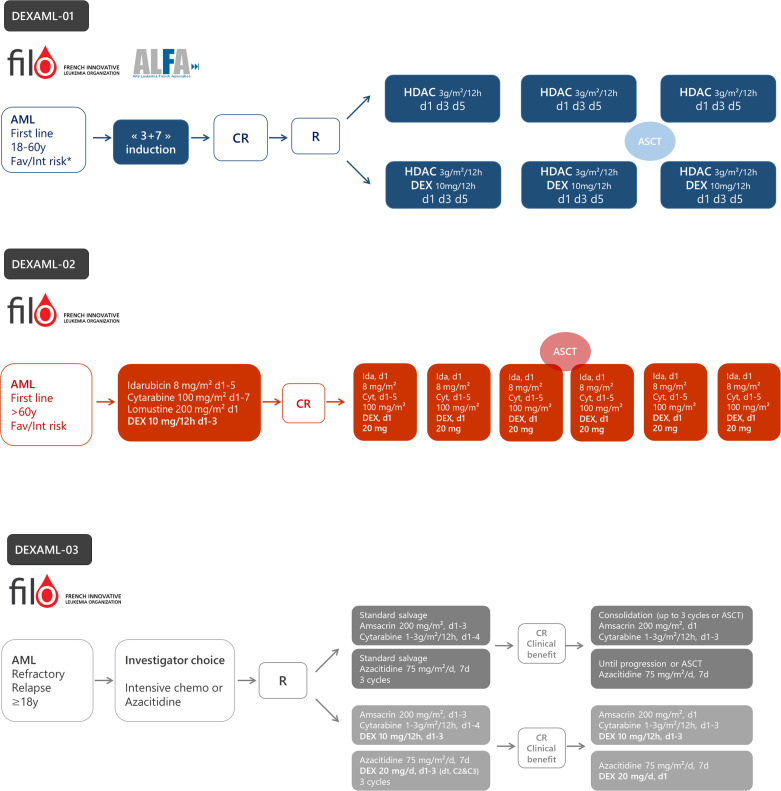
Figure 4.
The design of ongoing prospective trials to evaluate dexamethasone in acute myeloid leukemia (AML). DEXAML-01: patients (18–60 years) with favorable (fav) or intermediate (int) risk in first complete response after induction chemotherapy. DEXAML-02: patients > 60 with favorable or intermediate risk in first line treatment. DEXAML-03: patients > 18 years with refractory or relapsed AML. CR, complete response; R, randomization; HDAC, high dose cytarabine (3 g/m²/12 h on day 1, 3, and 5); DEX, dexamethasone.