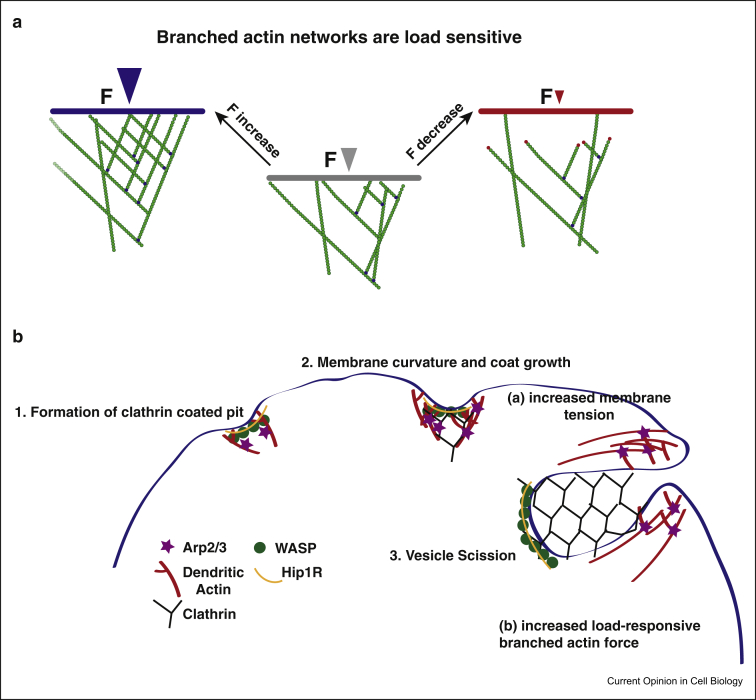
Figure 1.
Branched actin networks are load-sensitive and direct membrane dynamics. (A) Branched actin networks are load-sensitive. Scheme shows actin filaments growing at steady state with intermediate force load (middle, grey arrow). An increase in load (left, blue arrow) increases filament density, whereas after a decrease in load (right, red arrow) density decreases and branched filaments proximal to the membrane are preferentially capped (red). The Arp2/3 complex is highlighted in blue. F; Force. Figure adapted from the study by Mueller et al. [16]. (B) Spatially constrained branched actin networks enable endocytic uptake of clathrin-coated pits. Generation of force by the actin assembly shapes the dynamics of plasma membrane. This is especially evident by the manifestations of the actin cytoskeleton during clathrin-mediated endocytosis (CME). Actin is organised as a radial branched array with growing ends facing the base of the pit. During endocytosis, long actin filaments bend between attachment sites in the coat and the base of the endocytic vesicle, and therefore endocytic internalisation depends on elastic energy stored in bent filaments. This is based on the neck of the endocytic vesicle being a flexible spring and generating tension between the plasma membrane and the nascent vesicle. It has been shown that a band of the actin capturing molecule Hip1R near the internal distal end of the vesicle could capture actin filaments and allow load-responsive polymerisation of Arp2/3-induced dendritic networks to oppose the membrane tension forces. Increased membrane tension directs more growing filaments toward the base of the pit increasing actin nucleation and bending locally and therefore increased force production derived from branched actin. Spatially constrained actin filament assembly therefore enables endocytosis [21].