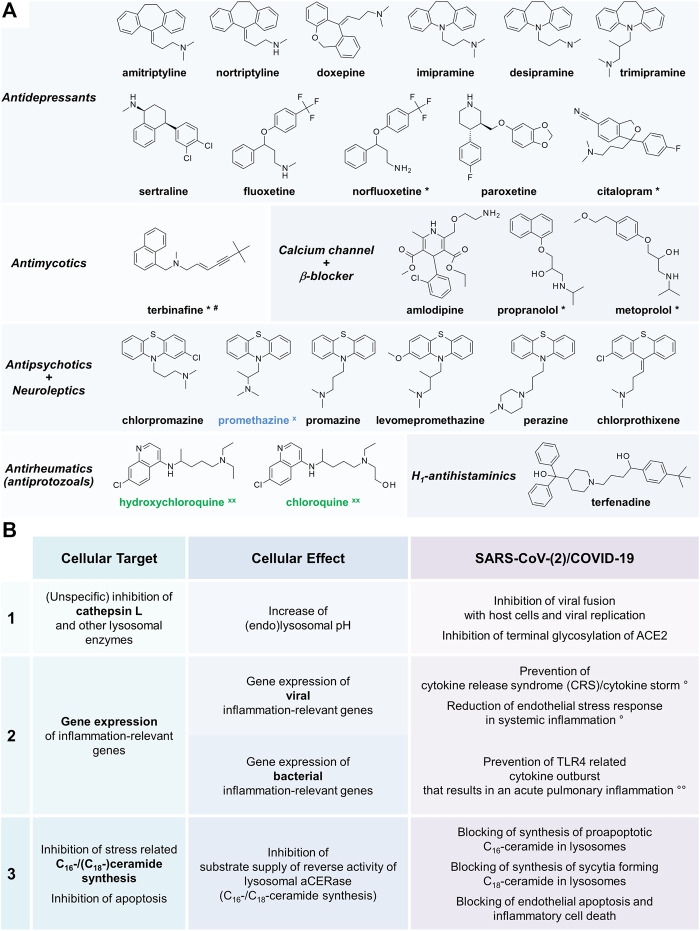
FIGURE 1.
(A) Variety of approved lysosomotropic compounds for various indications (Kornhuber et al., 2008; Blaess et al., 2018). Achievement of the desired lysosomotropic effect depends on the active compound, the dosage, and accumulation in lysosomes. Unless indicated, maximum daily doses are split into three applications. *Lysosomotropism very likely, but not yet confirmed, lysosomal drug concentration (effect) within the therapeutic margin expected; dosage: #single dose per day; x in vitro anti-SARS‐CoV tested, xx in vitro anti-SARS-CoV and anti-SARS-CoV-2 tested (Vincent et al., 2005; Kornhuber et al., 2008; Dyall et al., 2014; Zhou et al., 2016; Blaess et al., 2018; Liu et al., 2020; Weston et al., 2020). (B) Cellular targets, cellular effects, and effects related effects of lysosomotropic active compounds in SARS-CoV-2 infection/COVID-19 (Vincent et al., 2005; Masters, 2006; Mingo et al., 2015; Zhou et al., 2016; Blaess et al., 2018; Varga et al., 2020; Zhou et al., 2020). Lysosomotropic compounds target in mammalian cells three major targets related to SARS-CoV-2 infection/COVID-19: cathepsin L (1), gene expression of inflammation-relevant genes (2), C16-ceramide and C18-ceramide synthesis, and apoptosis of host cells (3). Addressing targets 1–3 results in various disease process interfering effects supposed to improve SARS-CoV-2 infection/COVID-19 outcome; (°) in viral infection and bacterial superinfection, (°°) only in bacterial superinfection.