FIGURE 2.
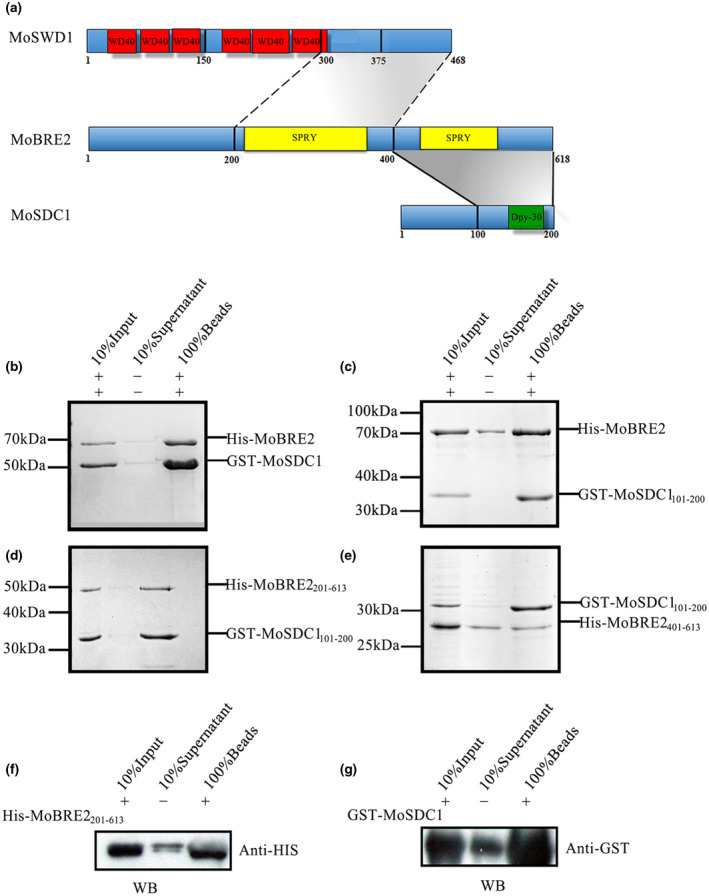
MoBre2 interacts directly with MoSdc1 in vitro. (a) Domain organization of MoBre2, MoSdc1, and MoSwd1. In MoSwd1, the WD40‐repeat domain is shown in red; in MoBre2, the SPRY domain (SPLA and RY anodine receptor domains) is shown in yellow; in MoSdc1, the DPY30 domain is shown in green. Numbers indicate residue numbers at the boundaries of various subdivisions. Protein interactions are indicated with grey areas. (b) Glutathione‐S‐transferase (GST) pull‐down assay with MoBre2 and MoSdc1. GST‐tagged MoSdc1 was incubated with His‐tagged MoBre2. The eluates were subjected to SDS‐polyacrylamide gel electrophoresis. (c, d) Full‐length MoBre2 interacts with the C‐terminal region of MoSdc1 (residues 101–200) but not with the N‐terminal region of MoSdc1 (residues 1–100). (e) The fragments of MoSdc1 (residues 101–200) interact with the two deletion mutants (MoBre2201–613, MoBre2401–613). (f, g) Western blot analysis shows that His‐tagged MoBre22 01–613 interacts with GST‐tagged full‐length MoSdc1
