Figure 1.
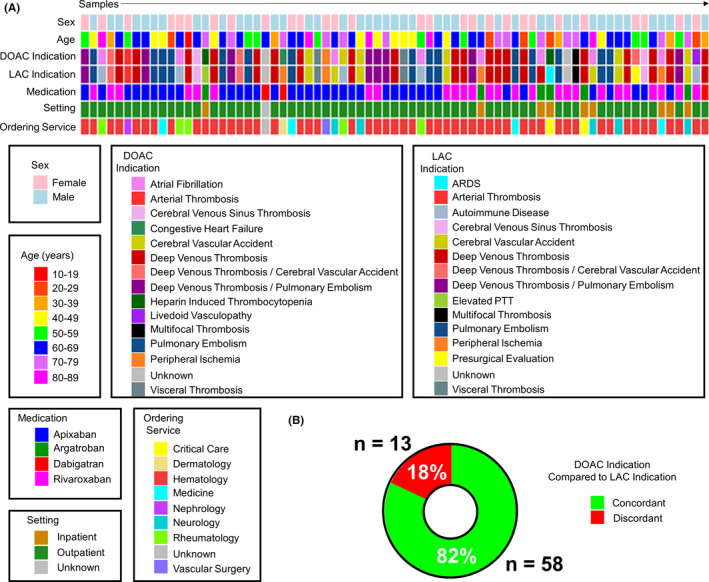
Sample characteristics of the unknown study cohort. (A) Heat map displaying each patient contributing a sample to our test cohort, their sex, age (by decade), the indication for anticoagulation therapy (DOAC indication), the indication for the patient to be tested for a LAC, the direct factor inhibitor the patient was receiving when the sample was drawn (medication), whether the sample was collected while the patient was in‐hospital or an outpatient, and the service ordering the test for each patient (n = 73 samples). The color code for each category is depicted below. (B) A plot showing the number and percent of samples where the indication for DOAC therapy and LAC testing were the same (concordant, plotted in green) or different (discordant, plotted in red). Cases where the indication was unknown (n = 2 samples) were excluded from analysis. ARDS, acute respiratory distress syndrome; DOAC, direct oral anticoagulant; LAC, lupus anticoagulant antibody; PTT, partial thromboplastin time
