Figure 1. NCGENES 2 Study design.
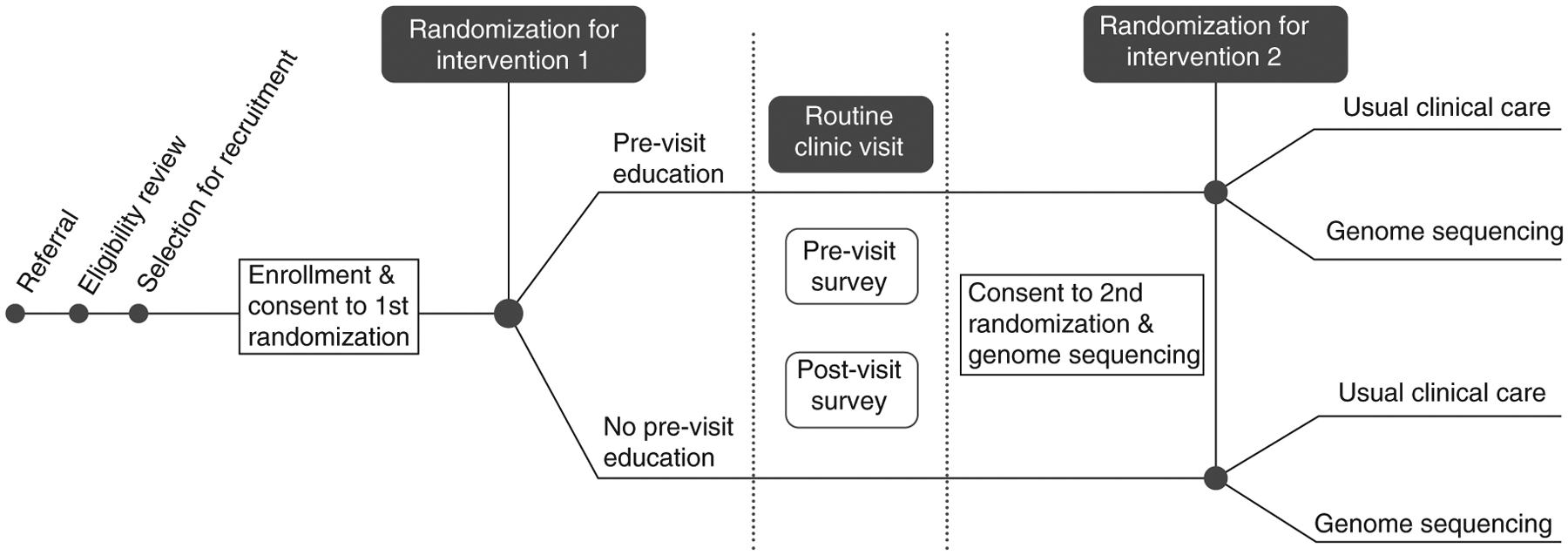
All eligible participants are new patients presenting for evaluation to pediatric genetics or pediatric neurology clinics. Enrollment is completed by phone prior to the scheduled new patient visit. The first randomization is +/− Pre-Visit Education materials which are mailed. Following the new patient visit, parents/participants consent/assent to the second randomization: +/− Genome Sequencing in addition to any tests their doctor ordered. Additional surveys occur after second randomization which are not depicted here. Further, the Pre-Visit Education arm receives a second mailed education packet focused on understanding test results.
