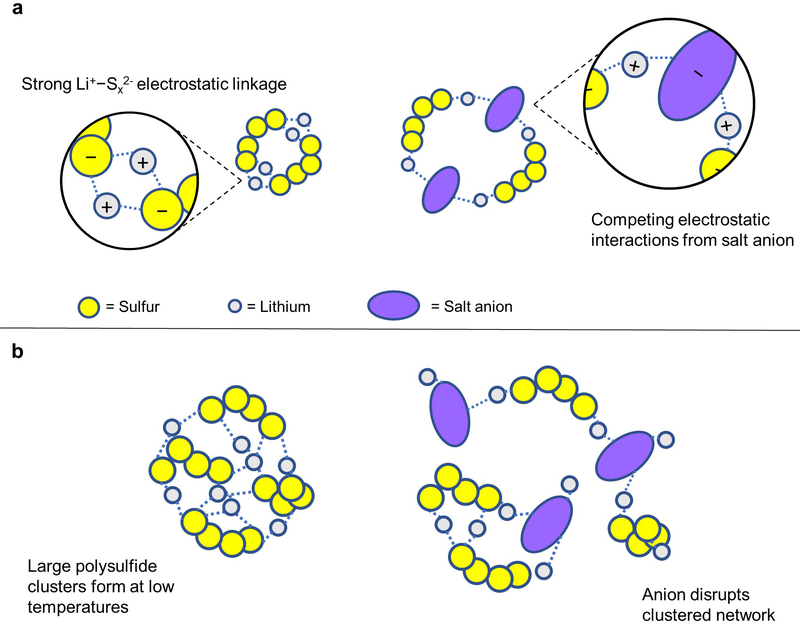
Figure 4.
Illustration of competing interactions between lithium species in solution. (a) Strong Li+−Sx2− bond networks can be disrupted from competing electrostatic interactions between lithium ions and lithium salt anions. (b) The lithium polysulfides that naturally form at low temperatures can be disrupted from the influence of competing lithium salt.