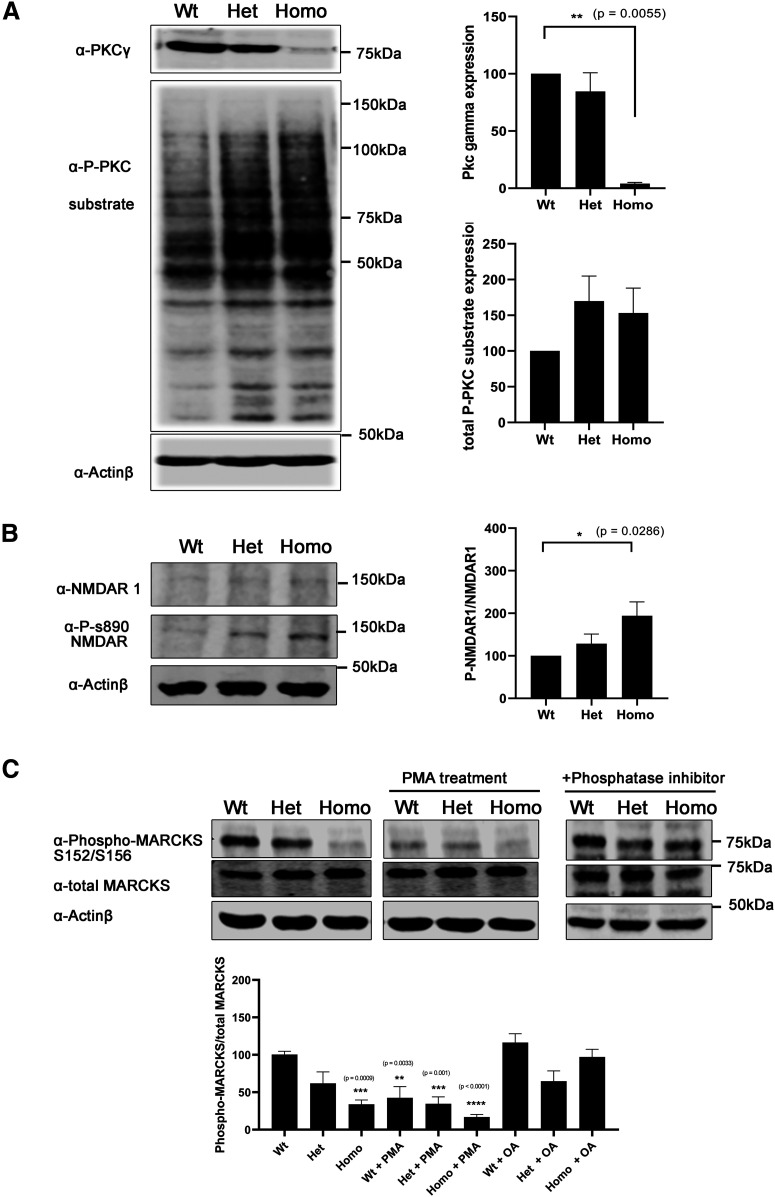
Figure 4.
PKCγ-A24E mouse shows highly PKC kinase activity. A, Western blot analysis of total PKCγ protein and phospho-PKC substrates from organotypic slice cultures. Phosphorylation of PKC substrates was increased in Het and Homo PKCγ-A24E mice. B, Western blot analysis normalized to actin shows PKCγ protein reduction in PKCγ-A24E mice, but phospho-PKC substrate is upregulated in PKCγ-A24E mice. NMDAR-S890 is known to be phosphorylated by PKCγ. Phospho-NMDAR-S890 protein expression is normalized to total NMDAR-N1 protein expression. This phosphorylation is increased in PKCγ-A24E mice. The data were normalized to Wt as 100% from three independent experiments. C, Phospho-MARCKS is a known substrate of PKC. Phospho-MARCKS S152/S156 compared with total MARCK was decreased in PKCγ-A24E mice and PMA-treated organotypic slice cultures from Wt mice (Wt = 100.4 ± 4.37%, n = 4; Het = 61.92 ± 15.25%, n = 4; Homo = 33.98 ± 5.83%, p = 0.0009, n = 4; Wt + PMA = 42.54 ± 15.4%, p = 0.0033, n = 4; Het + PMA = 34.58 ± 9.39%, p = 0.0010, n = 4; Homo + PMA = 16.89 ± 3.557%, p < 0.0001, n = 4). With added phosphatase inhibitor, the phospho-MARCKS reduction was partially rescued (Wt = 116.5 ± 11.8%, n = 3; Het = 64.68 ± 13.8%, n = 3; Homo = 97.15 ± 10.3%, n = 3). The two-tailed Mann–Whitney test was used to analyze the difference between each group, and Wt without any treatment is shown as 100%. Data are mean ± SEM.