Figure 5.
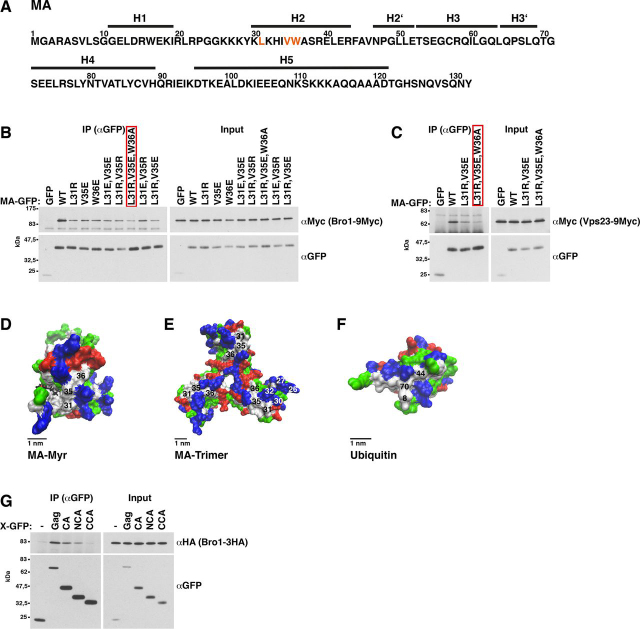
Mutation of an MA hydrophobic patch consisting of Leu-31, Val-35, and Trp-36 reduces Bro1 and Vps23 binding to MA; Bro1 binds via NCA to CA.A, MA protein sequence derived from pGag-EGFP (130) used as template in this study. Leu-31, Val-35, and Trp-36, the mutation of which reduced binding to Bro1, are marked in orange. Helix (H) assignments are derived from the MA X-ray structure (11). B and C, GFP-tagged MA versions were expressed from a 2µ vector with induced MET3 promoter in yeast cells carrying genomically 9Myc-tagged Bro1 or Vps23. MA was immunoprecipitated (IP) in the presence of 400 mm NaCl with anti-GFP antibodies, and coimmunoprecipitated Bro1 or Vps23 was detected by immunoblotting with anti-Myc antibodies. The red box indicates mutant MA3* that was chosen in subsequent experiments to diminish the ESCRT-MA interaction. B, Leu-31, Val-35, or Trp-36 mutation reduces the MA-Bro1 interaction. C, MA mutants that reduce the binding to Bro1 also diminish the MA-Vps23 interaction. D–F, molecular surface structures visualized with the VMD software. White, nonpolar aa; blue, basic aa; red, acidic aa; green, polar aa; black, myristoyl residue. D, MA NMR structure (PDB entry 2H3I) (19), showing that Leu-31, Val-35, and Trp-36 form a hydrophobic patch on the MA surface. E, MA trimer X-ray structure (PDB entry 1HIW) (11), showing that Leu-31, Val-35, and Trp-36 are located on the MA side that is exposed to the PM and are not part of the trimerization interface. Basic aa 26, 27, 30, and 32 were proposed to be involved in MA binding to phospholipids (13, 99, 101). F, human ubiquitin X-ray structure (PDB entry 1UBQ) (135), showing that the hydrophobic patch in MA consisting of Leu-31, Val-35, and Trp-36 resembles the hydrophobic ubiquitin patch consisting of Leu-8, Ile-44, and Val-70 which is involved in ubiquitin binding to Vps23, Bro1, TSG101, and ALIX (58, 67, 102). G, Bro1 coimmunoprecipitation with GFP-tagged CA versions, showing that Bro1 binds to the N-terminal CA domain; same as B except that GFP-tagged CA, NCA (aa 133–278), or CCA (aa 279–363) was expressed in a yeast strain carrying genomically 3HA-tagged Bro1 and that coimmunoprecipitated Bro1 was detected by immunoblotting with anti-HA antibodies.