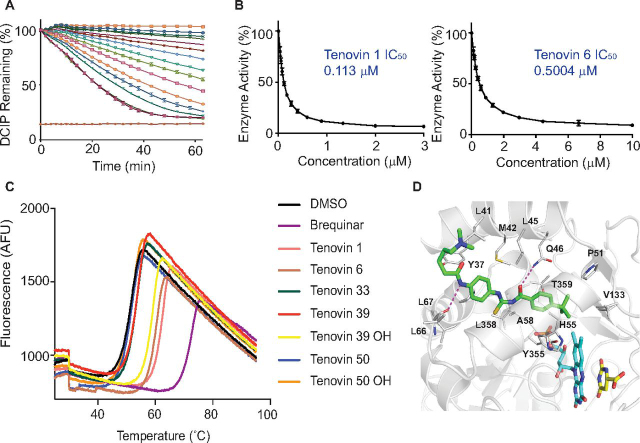
Figure 1.
Tenovins can inhibit DHODH.A, a dose titration of tenovin 1 in the enzymatic assay. Curves show the color reduction in DCIP over time for illustrative purposes. B, values obtained using a kinetic DHODH enzyme assay. Values correspond to the average of three independent repeats ± S.D. with three technical repeats each. C, thermal denaturation curve of DHODH incubated with various tenovins (200 μm) and brequinar (200 μm) as a positive control. D, co-crystal structure of tenovin 6 in complex with the enzyme DHODH. DHODH is shown as a cartoon (gray color), and the cofactor FMN (cyan carbons), the substrate DHO (yellow carbons), and bound tenovin 6 (green carbon) are shown as thick lines. The tenovin 6 binding pocket residues are denoted as thin lines, and the hydrogen bonds between DHODH and tenovin 6 are shown as dashed lines (magenta). Details of the crystal structure can be found in Table S1.