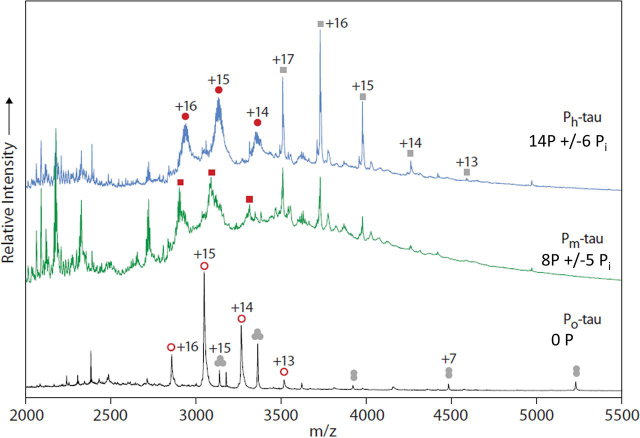
Figure 2.
Analysis of hTau-2N4R expressed in Sf9 cells by native MS. 20 µm of purified proteins in 200 mm ammonium acetate, pH 7.6, were analyzed by nanoflow electrospray ionization quadrupole TOF MS. Representative mass spectra for the highly phosphorylated states Ph-Tau (top) and Pm-Tau (middle) and unphosphorylated. P0-Tau (bottom) are shown. Top, main signals were assigned to charge state series of Ph-Tau (molecular mass of 46,883 Da; filled red circles) and a copurified component with a molecular mass of 59,642 Da (gray squares). Middle, spectrum of Pm-Tau displaying peaks assigned to a series of equivalent charge states but shifted toward lower m/z compared with those for Ph-Tau (red squares). Further signals match closely with those in the spectrum of Ph-Tau. Bottom, spectrum of control Tau P0 consisting of a series of charge states indicating a molecular mass of 45,724 Da (open red circles). A further series was assigned to a molecular mass of 47,085 Da. It was attributed to a trimeric species (gray circles) because it decomposes upon increasing collisional activation into highly charged (centered at +9, below m/z 2,000) monomeric and charge-stripped dimeric species (charges +6 to +8). Its monomeric mass of 15,691 Da matches the theoretical mass of the E. coli chaperone protein skp (see Table S4), taking into account removal of its N-terminal 20–amino acid signal peptide.