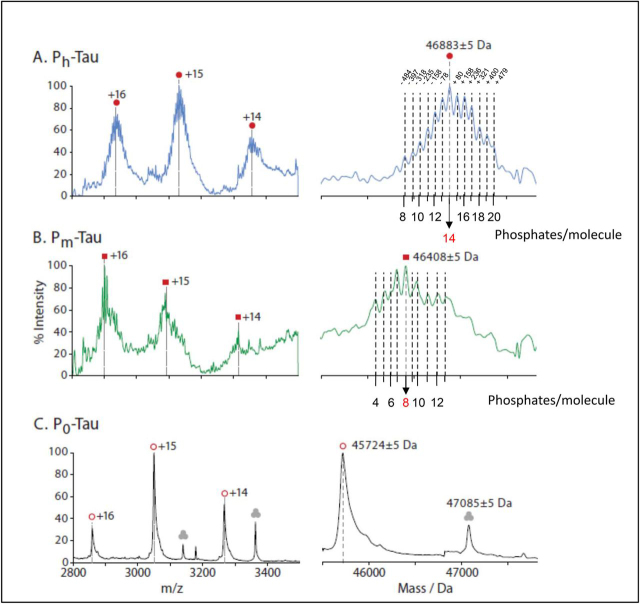
Figure 3.
Phosphorylation of hTau-2N4R expressed in Sf9 cells analyzed by native MS.Left, signals in the range between 2800 and 3500 m/z attributed to Tau proteins. Right, corresponding charge state–deconvoluted spectra. A, the mass spectrum of Ph-Tau (top) displays a center mass of 46,883 Da (filled red circles) and additionally six peaks on both sides equally spaced by ∼80 Da. B, Pm-Tau (middle) displays peaks of similar width shifted toward lower m/z compared with those for Ph-Tau, which can be assigned to the equivalent charge states and indicate a molecular mass of ∼46,408 Da (red squares). C, the spectrum of control Tau P0 consists of a series of charge states resulting in a comparatively sharp peak at a mass of 45,724 Da (45,850 −131Da ± 5 because of cleavage of first residue Met-1; open red circles). A further series was assigned to a molecular mass of 47,085 Da and was attributed to a trimeric species (gray circles) because it decomposes upon increasing collisional activation into highly charged (centered at +9, below m/z 2000) monomeric and charge-stripped dimeric species (charges +6 to +8). Its monomeric mass of 15,691 Da matches the theoretical mass of the E. coli chaperone protein skp (see Table S4), taking into account removal of its N-terminal 20–amino acid signal peptide.