Fig. 4.
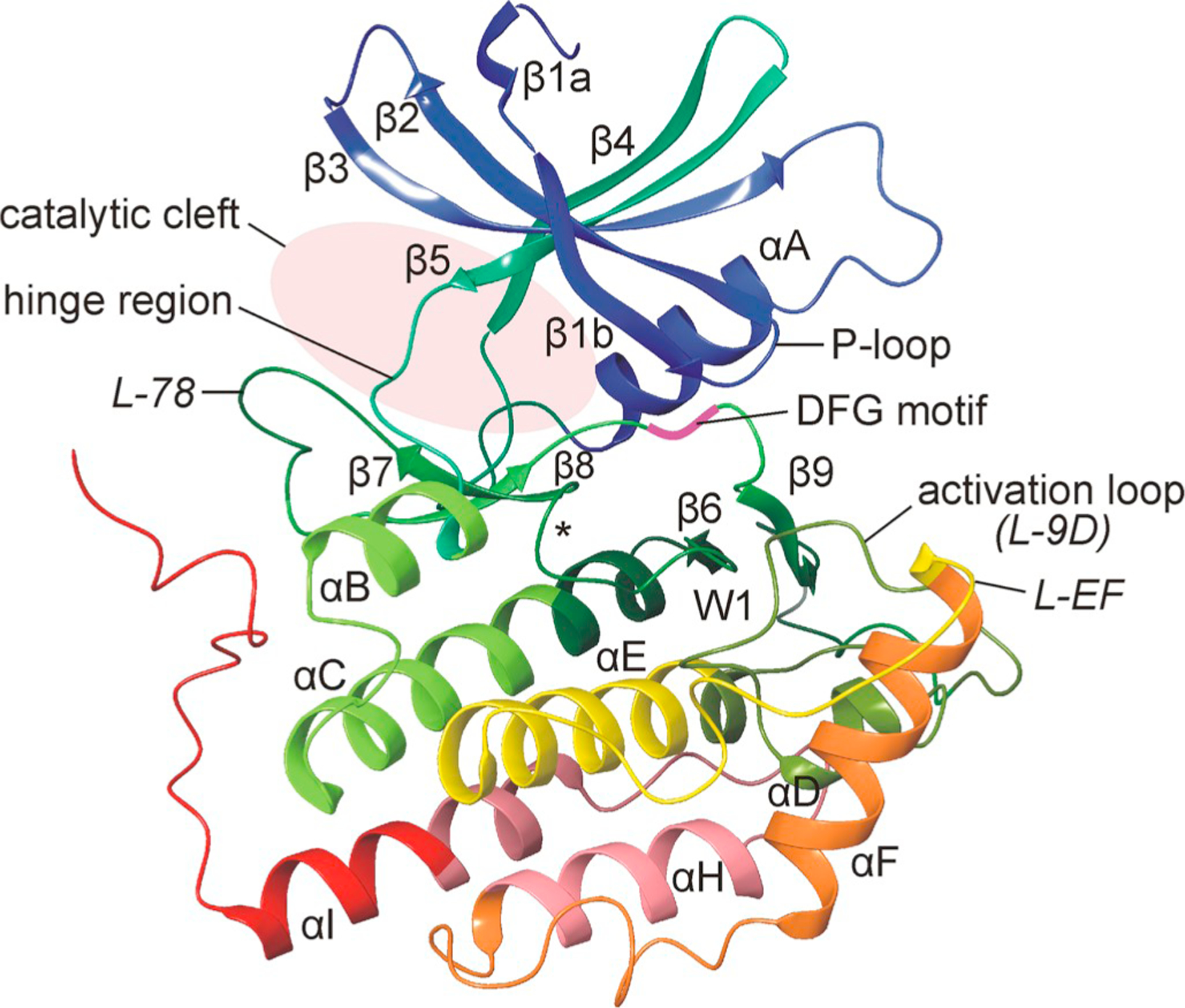
Three-dimensional structure of human CK1δ. Representation of the three-dimensional structure of human CK1δ. The structure of the N-lobe mainly consists of β-sheet strands while the larger C-terminal lobe is mainly composed by α-helices and loop structures. Structural elements are labeled according to Xu et al. (1995). Domains and residues of functional importance are labeled accordingly. Within loop L-89 the DFG motif is located with its aspartate residue being crucial for kinase activity and enzymatic function. Identification of a tungstate binding domain, indicated by W1, led to the identification of a recognition motif for the binding of phosphorylated substrates. The position of the catalytic loop (L-67) is marked with the asterisk (Xu et al., 1995; Longenecker et al., 1996). The figure was created by using CK1δ crystallization data deposited in the protein data bank (PDB) with ID 6GZM (Minzel et al., 2018).
