Figure 3.
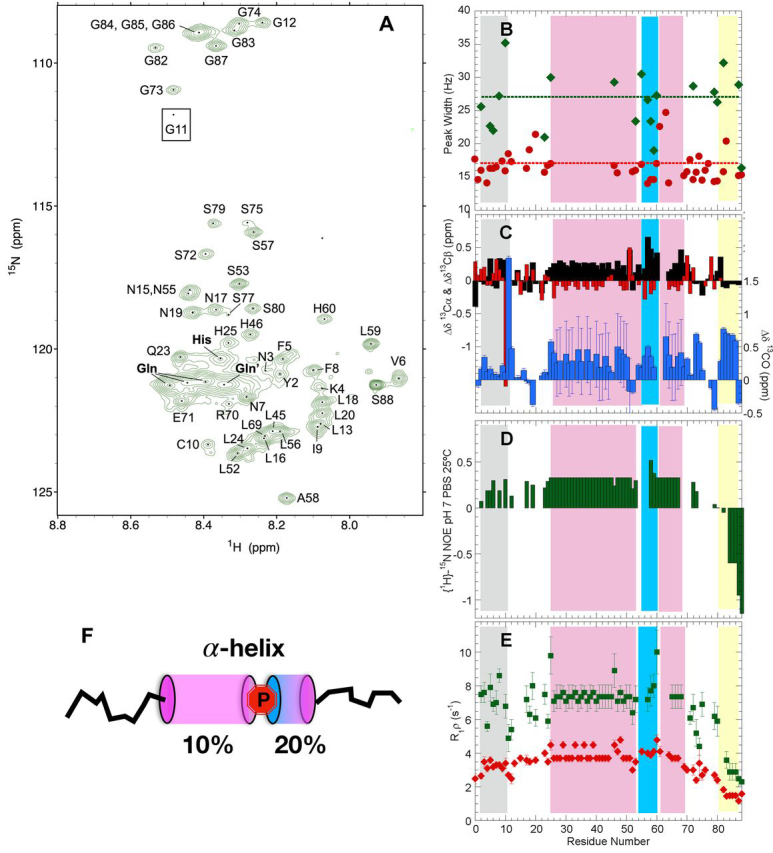
The Q/H-stretch of Orb2A partially adopts α-helical conformations and rigidifies at pH 7.B–E, the different zones of the Orb2 PLD are shaded as follows: N-terminal hydrophobic stretch, gray; Q/H-rich, light magenta; residues 55–60, blue; H·Q-rich, light magenta; G-rich, yellow. In the Q/H or H·Q-rich regions most of the values shown come from averaging of overlapped His or Gln peaks. A, 2D 1H-15N HSQC spectrum of Orb2A PLD registered at pH 7.0, 25°C. The bold His, Gln, and Gln′ labels refer to overlapped His and Gln peaks; these arise from residues in the amyloidogenic segment. The Gln′ peak arises from Gln, which follows His along the sequence. The signal of Gly-11 (boxed) is beneath the lowest selected contour level. To afford a fair appreciation of the wider peak widths observed here relative to the spectrum at pH 4, the same axes, aspect ratio, and multiplication factor between contour levels (1.4) are used here as in Fig. 2A. B, 1H peak width of Orb2 PLD 1H-15N HSQC peaks at 25°C and pH 7 (green diamonds) and pH 4 (red circles). The dotted lines represent the average peak widths at pH 7 (green) and pH 4 (red). Overlapped peaks are excluded. The uncertainties are approximately ± 1.5 Hz. C, conformational chemical shifts for 13Cα (black bars), 13Cβ (red bars), and 13CO (blue bars) at pH 7.0, 25°C. For clarity, the y axis scale is shifted for the 13CO Δδ values. The 13CO of Gly-11 and the 13Cβ of Cys are outliers. The 13CO Δδ values of the Q-rich region have large uncertainties because of the broad nature of the overlapped peak of Q residues. D, fast ps/ns dynamics assessed by the heteronuclear {1H}-15N NOE. Uncertainties are about ± 0.015. E, transverse relaxation rates in the rotating frame (R1ρ) for Orb2 PLD at pH 7.0, 25°C (green squares). For comparison, the R1ρ value obtained at pH 4.0, 25°C and already shown in Fig. 3C are also shown here by red diamonds. F, at pH 7, the Q/H segments (magenta cylinders), and the 55–60 segment (blue cylinder) adopts a small but detectable population of α-helical conformers separated by proline 54 (red).
