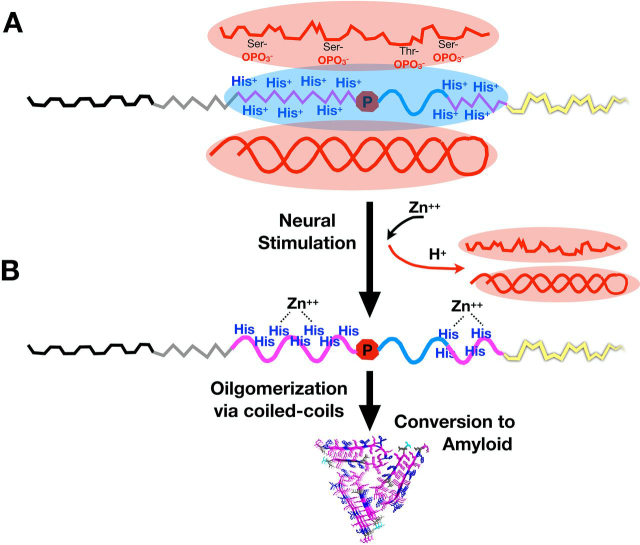
Figure 5.
His neutralization unleashes α-helix formation, oligomerization and amyloidogenesis.A, the first N-terminal hydrophobic segment (black) may interact with membranes which promote partial α-helix formation as reported by Soria et al. (12). The proximity of phosphorylated Tob protein (top, red), RNA (bottom, red), and/or possibly other polyanions, creates a negatively charged milieu which favors the cationic form of the 12 His residues (His +) of the Q/H-rich segments (magenta). This keeps the Q/H-rich segments disordered. Pro-54 (red hexagon) would also act to prevent premature amyloidogenesis. The 55–60 residue segment (blue) adopts a partial α-helix whereas the G/S-rich segment (yellow) remains disordered and flexible. B, following neural stimulation, the entry of Zn2+ and other cations could displace H+ and bind to the neutral form of His residues (blue) as proposed by Bajakian et al. (24); releasing Tob and RNA (red). The neutral form of His allows partial α-helix formation of the Q/H-rich segments, unlocking pathways to amyloid formation through coiled-coil intermediates.