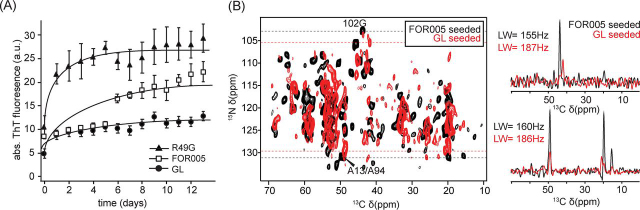
Figure 2.
Biophysical and NMR characterization of FOR005 VL variants.A, Seeded ThT aggregation kinetics of patient protein FOR005, protein containing the single point mutation R49G and for protein coding for the germline sequence. In all cases, 5% seeds were added to the monomeric protein and incubated at 37 °C. B, Comparison of germline and patient fibrils both seeded with ex-vivo seeds. Superposition of the 2D NCACX correlation spectra for patient (black) and germline (red) fibrils. In both cases, ex-vivo seeds have been employed. For G102 and A13/A94, 1D traces were extracted along the 13C dimension (right). Fibrils formed by the germline protein show significantly reduced sensitivity and have increased line width (FWHM = 187 Hz for germline, FWHM = 155 Hz for patient fibrils).