Figure 3.
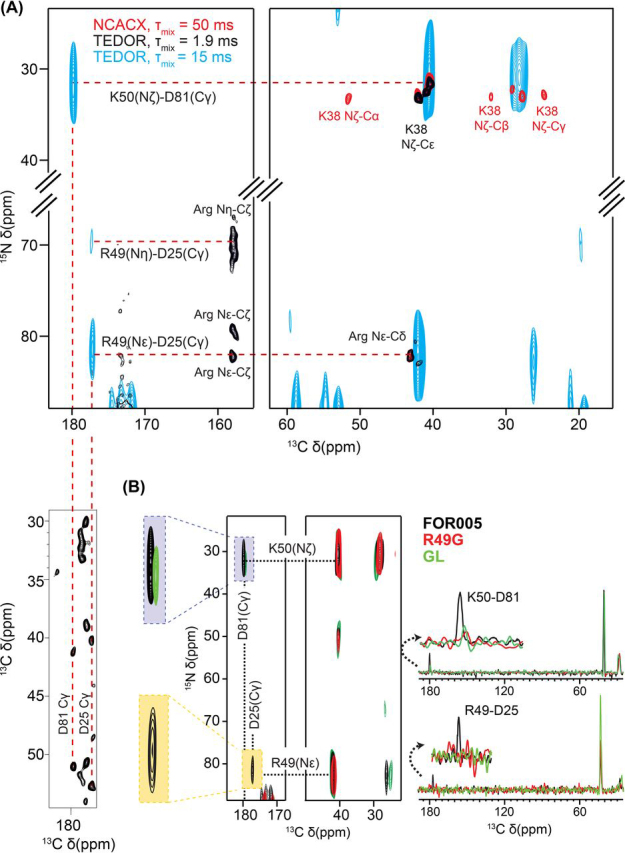
Electrostatic interactions between charged side chains in FOR005 patient, germline and R49G fibrils probed by TEDOR experiments.A, FOR005 patient fibrils. Superposition of 2D TEDOR spectra obtained with short (black, τmix= 1.9 ms) and long mixing times (cyan, τmix= 15 ms), focusing on the side chain resonances of arginine and lysine. The spectra are superimposed onto a 2D 13C,15N NCACX spectrum (red, τmix= 50 ms). We observe long-range contacts between lysine/arginine and aspartic acid side chains. The assignment of the carboxylic acid groups is indicated with a red dashed line in the 2D 13C,13C PDSD spectrum. B, Comparison of 2D TEDOR fibril spectra for FOR005 sequence variants. Patient fibril spectra are represented in black, germline fibrils in green and R49G fibrils in red. On the right, 1D rows extracted from the 2D TEDOR experiments are shown, illustrating the peak intensities for the different protein variants. In all cases, experiments were recorded under identical conditions with an equal number of scans and increments. Apparently, only fibrils formed by the patient protein FOR005 contain the salt bridges, whereas no cross-peaks are observed in R49G. For germline fibrils, a very weak peak seems to indicate a strongly reduced interaction involving K50-D81.
