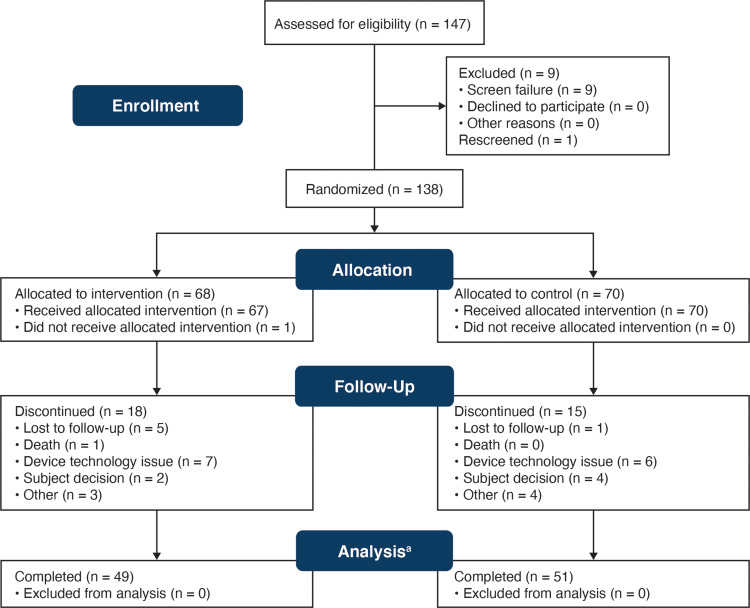
Figure 3.
CONSORT flow diagram.
Note:aThe n = 100 patients who completed the study also comprised the per-protocol analysis set, which consisted of all patients who met all eligibility criteria and were screened, randomized, and took ≥1 inhalation of study budesonide/formoterol, with ≥60 days of device time on study and without any major protocol deviations.
Abbreviation: CONSORT, Consolidated Standards of Reporting Trials.