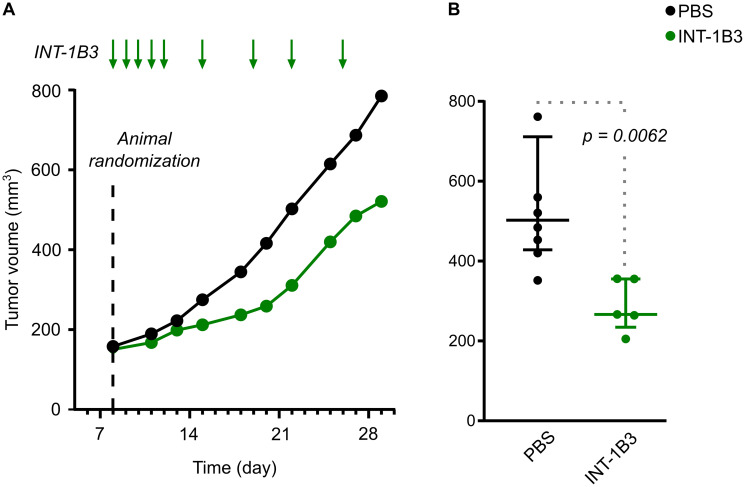
Figure 6. Effect of INT-1B3 on subcutaneous human melanoma A2058 tumor growth in immune-compromized mice.
Human melanoma A2058 tumor cells (5 × 106) in 0.1-mL PBS were injected subcutaneously in the flank BALBc/nude mice. After eight days, animals were randomized to experimental groups based on subcutaneous tumor size (median ~150 mm3; established subcutaneous tumor model). Animals were then treated with either vehicle (PBS, i.p.; black) or INT-1B3 (3 mg/kg/administration, i.v.; green) daily for five days the first week, then twice weekly for the next two weeks. Subcutaneous tumor volumes (expressed as mm3) were calculated throughout the study based on caliper-aided tumor measurements and applying the following formula: (length × width2)/2. (A) Time-dependent evolution of tumor volumes (median values expressed as mm3). (B) on Day 22, individual tumor volumes, median (horizontal bar) and interquartile range (vertical error bar), are presented for the indicated experimental groups. A 2-tailed non-parametric Mann–Whitney statistical analysis was performed for INT-1B3 (p = 0.0062) as compared to PBS-treated group, with n = 7 and 5 for PBS and INT-1B3 groups, respectively.