Figure 3.
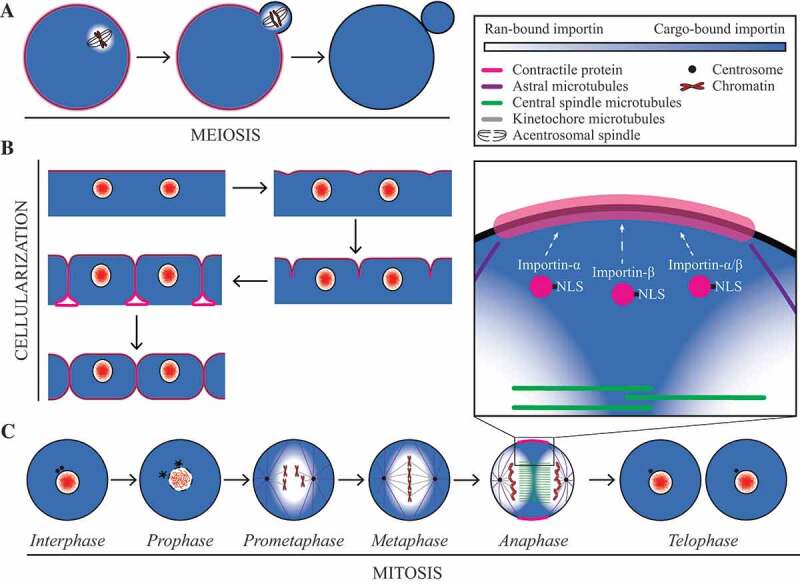
The Ran gradient regulates cortical proteins. (a) Cartoon schematics show an oocyte undergoing polar body extrusion. The legend describes the cell components for the cells in (a–c); contractile proteins (pink), astral microtubules (purple), central spindle microtubules (green), kinetochore microtubules (grey), centrosome (black) and chromatin (red). The Ran gradient is enriched around chromatin, which is positioned close to the cortex and functions as a molecular ruler to direct actin cap formation [47]. (b) During cellularization, Ran-GTP is sequestered in closed nuclei, and importin-binding could increase the localization of proteins at the cortex. The enrichment of proteins at the ingressing membrane would be directed by other factors. (c) In mitotic somatic cells Ran-GTP is generated at chromatin by RCC1 (RanGEF), which is hydrolysed by RanGAP in the cytosol to form a gradient. An inverse gradient of importin-bound proteins forms so that they are high near the cortex. In prometaphase and metaphase, Ran-GTP regulates spindle formation by releasing active spindle assembly factors from importin-binding [12]. In anaphase, importin-binding facilitates anillin’s localization to the equatorial cortex for cytokinesis, and we propose that other cortical proteins could similarly be regulated by importin-binding [1]. Ran-GTP is sequestered in the nucleus in telophase as the nuclear envelope reforms (Clarke & Zhang, 2008, 4)
