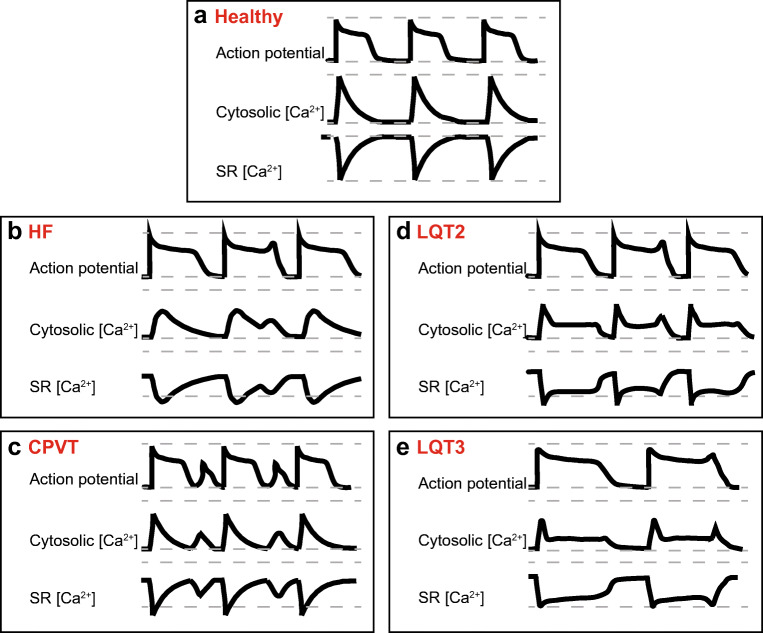
Fig. 2.
Comparison of proarrhythmic changes in action potentials and Ca2+ homeostasis in HF, CPVT, and LQTS 2 and 3 ventricular myocytes. a Schematic of action potentials, Ca2+ transients, and changes in intra-SR Ca2+ in a healthy human ventricular myocyte under β-adrenergic stimulation. Grey dashed lines indicate minimum and maximum Ca2+ levels reached in healthy myocytes. b In HF, APD is prolonged due to decrease in K+ currents and increase in late INa. Ca2+-dependent EADs/DADs underlie arrhythmogenesis under β-adrenergic stimulation. Enhanced sensitivity of RyR2 to intra-SR [Ca2+] due to increased phosphorylation and oxidation of the channel leads to termination of systolic Ca2+ release at reduced intra-SR [Ca2+]. Faster RyR2-mediated SR Ca2+ leak and reduced refractoriness of RyR2 also contributes to the enhanced propensity for proarrhythmic spontaneous Ca2+ release. Enhanced NCX1 activity, depressed SERCa activity and SR Ca2+ leak underlie reduced intra-SR [Ca2+] and diminished Ca2+ transient amplitude. Loss of dyadic contacts between T-tubular LTCCs and jSR RyR2s impedes Ca2+ transient rise. c Under β-adrenergic stimulation, CPVT myocytes exhibit spontaneous Ca2+ release via defective RyR2 complexes, leading to reduced Ca2+ transient amplitude and reduced intra-SR [Ca2+]. Posttranslational remodeling, mitochondrial dysfunction, and subcellular structural remodeling contribute to the hyperactivity of RyR2 caused by CPVT-associated mutations. Proarrhythmic activity of RyR2 drives NCX1 activity, causing a depolarizing inward current and DADs. Uncoupling of LTCCs and RyR2s due to dyad remodeling may increase Ca2+ transient rise time and reduce LTCC Ca2+-dependent inactivation which can result in longer APD. d In LQT2, loss-of-function mutation in KCNH2 reduces outward IKr and prolongs APD during β-adrenergic stimulation. SR Ca2+ leak is accelerated due to hyperphosphorylation of RyR2. SERCa-mediated SR Ca2+ uptake is accelerated at baseline due to PLB phosphorylation. Enhanced activity of hyperphosphorylated RyR2s contributes to a reduction of SR [Ca2+], Ca2+ transients amplitude, and arrhythmogenic EADs under β-adrenergic stimulation. e In LQT3, gain-of-function mutation in SCN5A increases inward late INa and prolongs APD. Arrhythmogenic activity occurs at rest, in the absence of β-adrenergic stimulation. Longer APD increases LTCC-mediated Ca2+ influx. Na+/Ca2+ overload and increased activity of SERCa due to PLB phosphorylation underlies increase in SR Ca2+ content, Ca2+ transient amplitude, and spontaneous RyR2-mediated Ca2+ release thereby EADs at slow rates