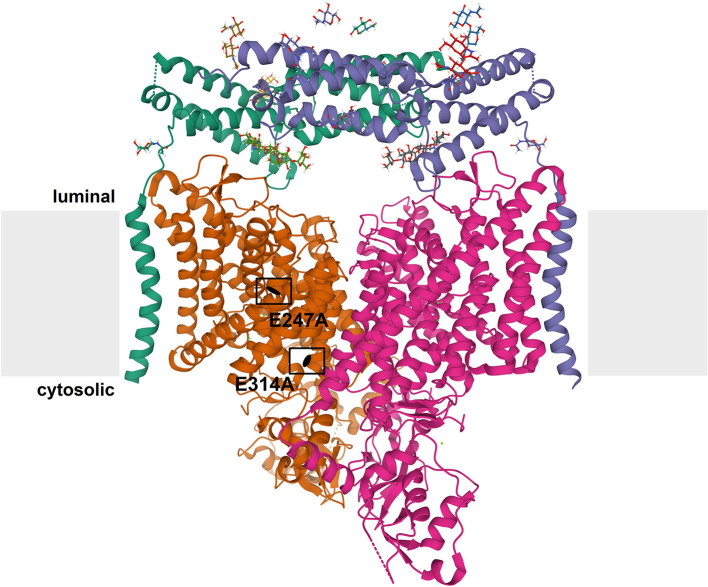
Figure 1.
Structure of CLC exchangers. The structure of human ClC-7 in complex with Ostm1 [Protein Data Bank [PDB]: 7JM7 (Schrecker et al., 2020)] viewed parallel to the membrane (depicted in gray) is shown as an example for the structure of CLC proteins. The subunits of the CLC homodimer are represented in orange and magenta. The globular CBS-containing domain of each subunit protrudes into the cytoplasm. Each CLC subunit provides an independent ion transport pathway. For one subunit, the positions of two key amino acids, the “gating glutamate” (E247 in ClC-7) and the “proton glutamate” (E314 in ClC-7), are indicated. The ClC-7 dimer binds two copies of its β-subunit, Ostm1, presented in green and blue. The heavily glycosylated Ostm1 is thought to shield ClC-7 from acidic proteases in the lysosomal lumen.