Figure 2.
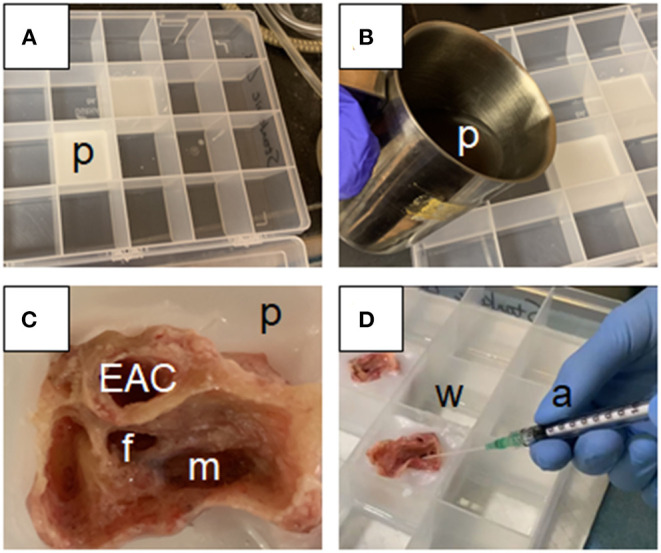
Experimental set-up for measurement of trans-tympanic drug delivery in situ: (A) Paraffin is heated to 60°C, poured into a clear plastic compartment organizer, and allowed to solidify to form a 1 cm deep paraffin base (p); (B) Once the base is solidified, additional hot paraffin (p) is added to an additional 1 cm depth; (C) The post-mastoidectomy temporal bone is set into the hot paraffin such that the external auditory canal (EAC) is oriented vertically, and becomes fixed in place as the cooling paraffin (p) forms a watertight shell surrounding the drilled mastoid (m), with access to the lateral surface of the tympanic membrane obtained via the EAC and to the medial surface via the facial recess (f); (D) Extra compartments within the compartment organizer are filled with water at 37°C to maintain humidity and consistent temperature throughout the experiment (w). A series of angiocatheters (a) are used to perform the following tasks throughout the experiment: filling of the middle ear space with normal saline via the antrum, applying drug formulation to the tympanic membrane via the EAC, and collecting middle ear aspirate via the facial recess.
