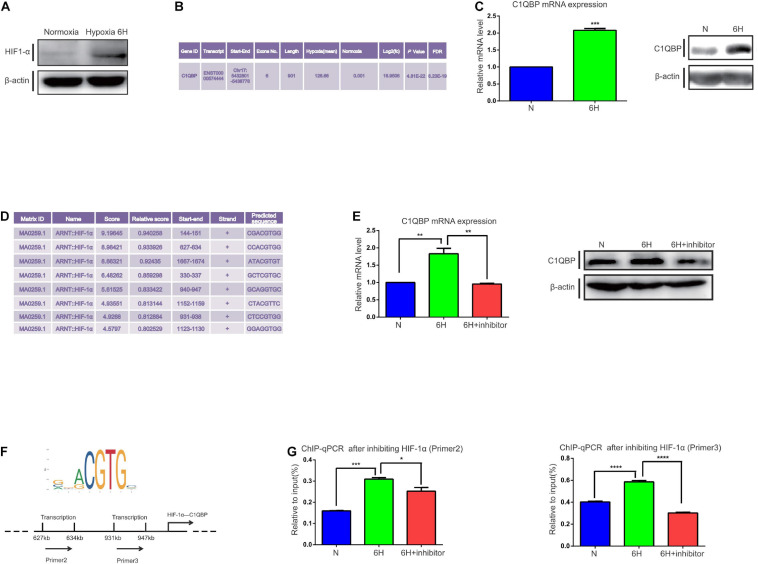
FIGURE 1.
Hypoxia-induced HIF-1α promotes C1QBP upregulation in TNBC. (A) MDA-MB-468 cells were exposed to 20% or 1% O2 for 6 h. Western blot shows the HIF-1α expression after 6 h of hypoxia; β-actin levels were used as the internal control. (B) Transcriptome analysis shows that C1QBP was upregulated during hypoxia. (C) MDA-MB-468 cells were exposed to 20% or 1% O2 for 6 h. Real-time qPCR and western blot were performed to determine the mRNA and protein levels of C1QBP; β-actin levels were used as the internal control. (D) Using the UCSC and JASPAR databases, 200–2,000 bp were selected downstream of the start site in the C1QBP promoter to show the potential binding sites for HIF-1α on C1QBP. (E) MDA-MB-468 cells were exposed to 20% O2, 1% O2, and 1% O2 with the HIF-1α inhibitor (10 μmol/ml) for 6 h, after which mRNA and protein levels were determined using real-time qPCR and Western blot; β-actin levels were used as the internal control. (F) The major binding site of HIF-1α on C1QBP (from the JASPAR database): two potential binding sites on the promoter of C1QBP. (G). MDA-MB-468 cells were exposed to 20% O2, 1% O2, and 1% O2 with the HIF-1α inhibitor (10 μmol/ml) for 6 h. ChIP was performed and the presence of the two binding sites was quantitatively determined using real-time qPCR. Three independent experiments were used to represent the mean ± standard deviation values. *P < 0.05, **P < 0.01, ***P < 0.0001, and ****P < 0.0001.