Abstract
This study analyzed the association between medication adherence and the intrapatient variability (IPV) of tacrolimus concentrations among kidney transplant recipients through a post hoc analysis of the dataset from a recently conducted randomized controlled trial. Among 138 patients enrolled in the original trial, 92 patients with ≥ 5 months of medication event monitoring system (MEMS) use and ≥ 4 tacrolimus trough values were included in this post hoc analysis. The variability of tacrolimus trough levels was calculated using coefficient variation (CV) and mean absolute deviation. Adherence was assessed using MEMS and self-report via the Basal Assessment of Adherence to Immunosuppressive Medication Scale. There were no statistically significant differences in the CV [median 16.5% [interquartile range 11.6–25.5%] and 16.0% [11.5–23.5%], respectively, P = .602] between the nonadherent (n = 59) and adherent groups (n = 33). There was also no significant correlation between the CV and adherence detected by MEMS (taking adherence, ρ = − 0.067, P = .527; dosing adherence, ρ = − 0.098, P = .352; timing adherence, ρ = − 0.113, P = .284). Similarly, adherence measured by self-report did not significantly affect the IPV (P = .452). In this post hoc analysis, nonadherent behavior, measured through electronic monitoring or self-report, did not affect the IPV.
Subject terms: Medical research, Nephrology
Introduction
Immunosuppressive (IS) agents are used for induction, maintenance, and reversal of established transplant rejection. Since the beginning of kidney transplantation, the IS regimen has continued to evolve; in particular, the use of tacrolimus has markedly increased. In 2017, the most common initial IS regimens were tacrolimus, mycophenolate mofetil (MMF), and steroids in 57.6% of recipients, followed by tacrolimus and MMF in 32.9%1. While the use of tacrolimus has many positive effects, it is difficult to maintain constant tacrolimus concentrations because multiple factors affect the pharmacokinetics and pharmacodynamics of tacrolimus. Key determinant factors include nonadherence, drug-drug interactions, timing and fat contents of meals, and genetic factors, such as cytochrome P450 (CYP) 3A5, CYP3A4, and adenosine triphosphate (ATP)-binding cassette sub-family B member 1 (ABCB1)2. Tacrolimus, therefore, is a commonly used medication with a narrow therapeutic window which is routinely monitored with therapeutic drug monitoring. Nonadherence to IS medication is known to have a large impact on graft outcome after kidney transplantation, and previous studies have reported a high rate (up to 65%) of nonadherence among kidney transplant recipients3,4.
Kidney transplant recipients with high intrapatient variability (IPV) of tacrolimus exposure are at higher risk of poor graft outcomes, including rejection and graft failure5,6. The high IPV of tacrolimus has been undoubtedly believed to be primarily the result of nonadherent behavior. It is also sometimes used as a surrogate marker for nonadherence in clinical studies7; a recent study suggested that high IPV can be considered a proxy measure of nonadherence8. However, it remains unclear how important nonadherence is in determining IPV or whether calculated IPV based on tacrolimus levels measured during outpatient visits reflects nonadherent behavior. This poor understanding partly results from the multifactorial nature of medication nonadherence and from the inherent limitations of clinical methods used to measure medication nonadherence. Nonadherence is defined as deviation from the prescribed medication regimen sufficient to adversely influence the regimen’s intended effect9. Clinical methods to measure adherence, such as pill counts, questionnaires, and patients’ diaries, can over- or underestimate adherence10. Instead, it has become widely accepted that electronic monitoring (EM) provides the best estimate of adherence11. The Food and Drug Administration endorsed “smart bottles” as a tool to improve drug development trials by confirming protocol fidelity. EM is an objective method of detecting nonadherence in nature and has been proven useful in quantifying medication adherence and associated clinical outcomes in prospective studies involving adult kidney transplant recipients12. In this context, data from clinical trials using EM of tacrolimus may provide the best opportunity to investigate the association between tacrolimus IPV and nonadherence.
Therefore, the aim of this study was to analyze the association between medication adherence and the IPV of tacrolimus concentrations among kidney transplant recipients, using the dataset from a recently conducted randomized controlled trial (RCT).
Results
Patient’s characteristics
A total of 92 kidney recipients were eligible for this post hoc analysis. The median values of CV and MAD for the total population were 16.4% (IQR 11.3–24.9%) and 13.1% (IQR 8.5–19.2%), respectively. The median age at transplant was 43 years (IQR 18–67), and the median post-transplant period was 1.8 years (IQR 1.1–3.7). There were 58 males (63.8%), and the mean body mass index was 22.0 ± 3.1 kg/m2. Thirty-seven (40.2%) recipients received kidneys from deceased donors, and 55 (59.8%) received kidneys from living donors. Regarding medication regimens, 86 (93.5%) patients received MMF, and 86 (93.5%) patients received prednisone. Using MEMS data, the nonadherent group was defined as < 98% taking adherence and/or at least one drug holiday, as described previously13. There was no difference in baseline characteristics between the adherent and nonadherent groups (Table 1). Taking adherence measured by MEMS was 99.7% (99.2–100.0), 86.0% (63.9–95.9), and 96.3% (77.7–99.5) in the adherent, nonadherent, and total study groups, respectively (P < 0.001).
Table 1.
Patient demographics.
| Total (n = 92) | Adherent (n = 33) | Non-adherent (n = 59) | P | |
|---|---|---|---|---|
| Age at transplant, year, median (IQR) | 43 (18–67) | 43 (21–67) | 43 (18–58) | .078 |
| Length of time post-transplant, year (IQR) | 1.8 (1.1–3.7) | 1.7 (1.1–3.2) | 1.8 (1.1–3.9) | .663 |
| Sex, male (%) | 58 (63.0) | 22 (66.7) | 36 (61.0) | .590 |
| BMI, mean (SD), kg/m2 | 22.0 (3.1) | 21.9 (3.3) | 22.1 (3.0) | .801 |
| Education (%) | .828 | |||
| Undergraduate college | 46 (50.0) | 16 (48.5) | 30 (50.8) | |
| Graduate college | 46 (50.0) | 17 (51.5) | 29 (49.2) | |
| Donor type (%) | .904 | |||
| Deceased | 37 (40.2) | 13 (39.4) | 24 (40.7) | |
| Living | 55 (59.8) | 20 (60.6) | 35 (59.3) | |
| Immunosuppressive (%) | ||||
| MMF | 86 (93.5) | 30 (90.9) | 56 (94.9) | .455 |
| Prednisone | 86 (93.5) | 31 (93.9) | 55 (93.2) | .893 |
| Daily tacrolimus dose, mean ± SD, mg | ||||
| At enrollment | 4.5 ± 2.4 | 4.1 ± 1.7 | 4.7 ± 2.6 | .229 |
| At last visit | 4.1 ± 2.1 | 3.9 ± 1.7 | 4.3 ± 2.3 | .292 |
| Trough concentration (C0) of tacrolimus, mean ± SD, ng/mL | ||||
| At enrollment | 5.8 ± 1.8 | 6.4 ± 2.2 | 5.4 ± 1.5 | .008 |
| At last visit | 5.8 ± 1.6 | 6.0 ± 1.5 | 5.7 ± 1.7 | .302 |
| MEMS, median (IQR), % | ||||
| Taking adherence | 96.3 (77.7 – 99.5) | 99.7 (99.2 – 100.0) | 86.0 (63.9 – 95.9) | < .001 |
| Dosing adherence | 89.7 (65.2 – 97.0) | 97.8 (95.9 – 99.5) | 72.5 (53.3 – 89.8) | < .001 |
| Timing adherence | 90.9 (61.6 – 98.0) | 98.1 (96.5 – 99.3) | 73.5 (50.0 – 90.1) | < .001 |
| TTR, median (IQR) | 47.7 (17.5 – 76.5) | 50.1 (17.5 – 82.3) | 43.1 (14.9 – 71.0) | .552 |
IQR, interquartile range; BMI, body mass index; SD, standard deviation; MMF, mycophenolate mofetil; MEMS, medication event monitoring system; TTR, time in therapeutic range.
Differences in IPV indices between adherent and nonadherent groups
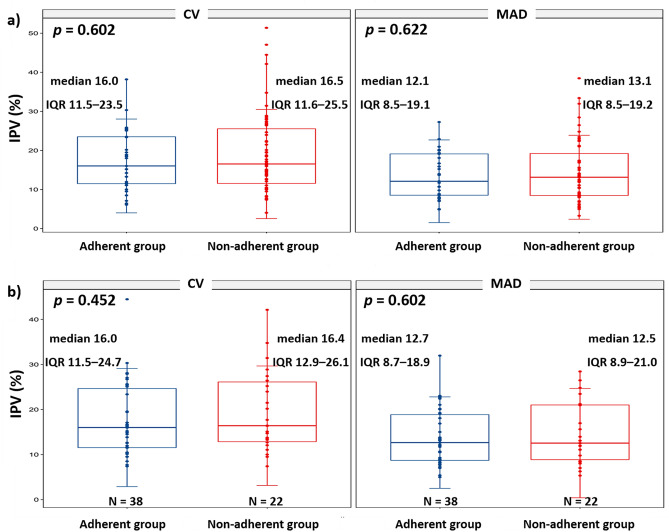
There were no statistically significant differences in either the CV (median 16.5 [IQR 11.6–5.5] and 16.0 [11.5 23.5], respectively, P = 0.602) or the MAD (median 13.1 [IQR 8.5–19.2) and 12.1 [8.5–19.1], respectively, P = 0.622] between the nonadherent group (n = 59) and the adherent group (n = 33) (Fig. 1a). Furthermore, the IPV was not different between groups defined by various cutoff values of taking adherence (Supplementary Table S1).
Figure 1.
Differences in IPV indices between adherent and nonadherent groups measured through an electronic monitoring system (a) and self-report (b). CV, coefficient variation; IPV, intrapatient variability; IQR, interquartile range; MAD, mean absolute deviation.
The median value of TTR in the total population was 47.7% (IQR 17.5–76.5). Like CV and MAD, there were no statistically significant differences in the TTR (median 50.1 [IQR 17.5–82.3] and 43.1 [14.9–71.0], respectively, P = 0.552) between the two groups.
In adherence measured through self-report, over time, adherent patients gradually decreased (28 days, 62; 90 days, 49; 180 days, 46), and non-adherent patients gradually increased (28 days, 30; 90 days, 43; 180 days, 46). Three self-report checks during the 6-month follow-up revealed that 38 patients were all adherent, and 22 patients were all non-adherent. There were no significant differences in the IPV indices between these two groups (CV, P = 0.452; MAD, P = 0.602) (Fig. 1b).
Correlation between IPV indices and adherence measured by MEMS
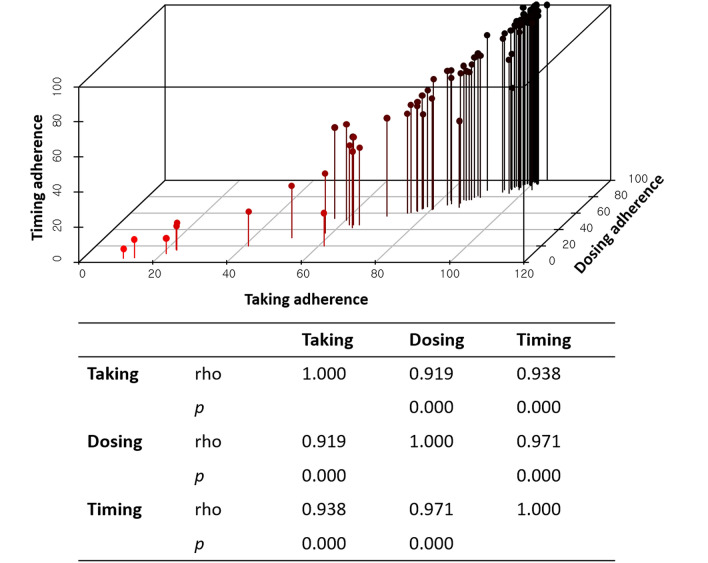
We analyzed the correlation between taking, dosing, and timing adherence. There was a linear correlation among the three parameters of MEMS (taking vs. dosing, rho = 0.919, P < 0.001; taking vs. timing, rho = 0.938, P < 0.001; dosing vs. timing, rho = 0.971, P < 0.001) (Fig. 2).
Figure 2.
Correlation between intrapatient variability indices and adherence measured by the medication event monitoring system.
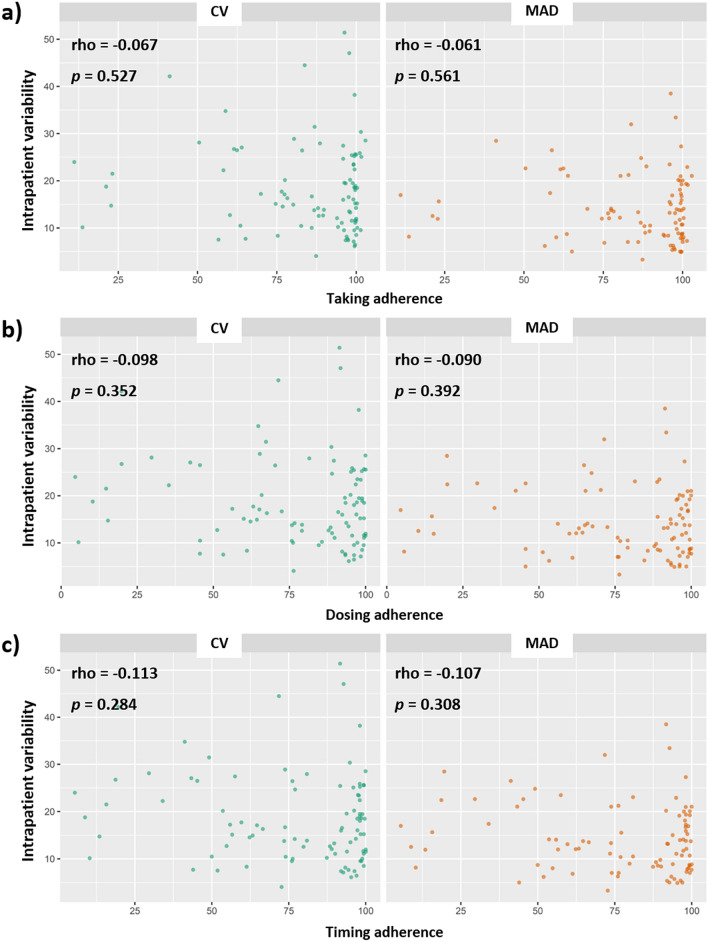
Correlation analysis using Spearman’s rho failed to find a significant correlation between longitudinal adherence measured by MEMS (taking adherence, ρ = − 0.067, P = 0.527; dosing adherence, ρ = − 0.098, P = 0.352; timing adherence, ρ = − 0.113, P = 0.284) and IPV indices. Figure 3 shows the results as a scatter plot.
Figure 3.
Correlation analysis using Spearman’s rho to identify correlations between adherence measures using the medication event monitoring system and intrapatient variability indices. Taking adherence (a), dosing adherence (b), timing adherence (c). CV, coefficient variation; MAD, mean absolute deviation.
Clinical outcomes
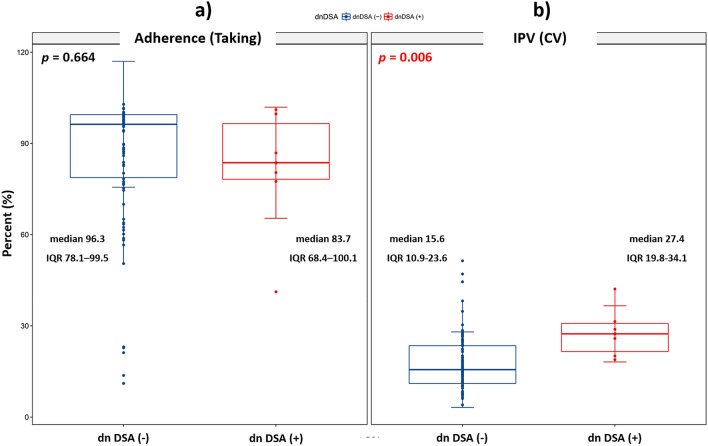
The development of dnDSA was found in six patients (two in the adherent group and four in the nonadherent group; P = 0.893). Taking adherence was not significantly different between the dnDSA-negative (median 96.3 [IQR 78.1–99.5]) and -positive groups (median 83.7 [IQR 68.4–100.1]) (P = 0.664) (Fig. 4a). However, the CV was significantly higher in the dnDSA-positive group (CV 27.4, IQR 19.8–34.1) compared to the dnDSA negative group CV 15.6, IQR 10.9–23.6) (P = 0.006) (Fig. 4b). Changes in the eGFR using the Modification of Diet in Renal Disease Study also did not differ between the two groups at 1, 3, and 5 years after enrollment in the study (Supplementary Table S2).
Figure 4.
The development of dnDSA in terms of taking adherence (a) and IPV (b). IPV, intrapatient variability; CV, coefficient variation; dnDSA, de novo donor-specific antibodies; IQR, interquartile range.
Discussion
In this study, we performed post hoc analysis to investigate the relationship between adherence and tacrolimus IPV using the dataset from the original RCT. As expected, high IPV was associated with dnDSA development, which was concordant with previous reports5,6. It has been assumed that nonadherent behavior may be related to high IPV; however, no literature had provided clear evidence until now. We used EM and defined nonadherence as < 98% taking adherence and/or at least one drug holiday, based on a previous study14. We also found no clear relationship between nonadherence and tacrolimus IPV in stable renal transplant patients. This finding is completely concordant with a report from the Leiden group15. We also attempted to compare various cutoff values (taking adherence 95%, 90%, 80%, 70%, and 50%) because we had concerns that the cutoff value of nonadherence was too high; however, we did not find any significant relationship. A recent study using dried blood spot technology also failed to show a clear relationship between nonadherence and tacrolimus IPV; they recommended that the correlations must be more closely revealed before IPV can be used as a surrogate marker of nonadherence16.
As reported in previous studies, tacrolimus IPV was significantly higher in the dnDSA-positive group in this study17,18. On the contrary, we could not find any association between adherence and dnDSA development in stable renal transplant recipients. Therefore, we need an alternate explanation for tacrolimus IPV instead of nonadherence.
Trough level collection time could be regarded as a cause of high IPV in outpatient clinics. However, high IPV caused by differences in blood collection time may not adversely affect graft outcome. The timing and fat contents of meals affect the likelihood of high IPV. Tacrolimus is absorbed by the small intestine, and bioavailability is about 25%4. Kimikawa et al. compared trough concentrations between preprandial and postprandial oral administration and found trough concentrations to be effectively absorbed by preprandial oral administration19. In addition, a high-fat meal reduces the rate of tacrolimus absorption relative to a low-fat meal20. Therefore, we always recommend that patients take tacrolimus on a gastric empty status (2 h after meals and 1 h before meals) based on hospital protocol. Drug-drug interaction (for example, proton pump inhibitors and calcium channel blockers) is also a key factor in increased tacrolimus IPV. Therefore, kidney recipients need to be careful when taking other medications21. Genetic factors may also affect the development of high IPV. Stifft et al. reported a decrease in IPV when converting tacrolimus to once-daily in stable renal transplant patients, especially in patients with the CYP3A5*1/3 genotypes22. Other studies have reported that CYP3A5 polymorphism affects the achievement of target tacrolimus trough levels and increases early acute rejection23.
Immunosuppressive medications are known to have a critical effect on outcome after kidney transplantation. Thus, the nonadherent behavior of recipients to IS has long been a concern for transplant specialists. Adherence to medication is generally defined as the extent to which patients take medications as prescribed by their health care providers24. Early declining medication nonadherence is associated with chronic nonadherence and eventually leads to adverse clinical outcomes25. Contrary to expectations, using a mobile medication management application did not contribute to the improvement of adherence rate (overall nonadherence rate: mobile group, 65% and control group, 62.1%, respectively; odds ratio, 1.14; P = 0.89). This was attributed to the early discontinuation of the mobile application and the failure to provide strategies to facilitate patient engagement with the application.
There were several limitations to our study. A 6-month period of follow-up for adherence may not sufficiently represent the patients’ adherence behavior. However, adherence usually peaks in the early period after transplantation and declines thereafter. Thus, a measured 6-month period of adherence 1 year post-transplant was a reasonable approach to investigate the possibility of a relationship between adherence and IPV and its effects on the long-term transplant outcome. In addition, our study population had a low IPV overall. In previous studies, a criterion affecting clinical outcome was presented with a CV value of 3016. In this study, only eight out of 92 had a CV > 30. The study population consisted of motivated patients from an RCT and was composed of a single ethnic group. Therefore, the results cannot be extrapolated to patients with different ethnicities in other regions. Lastly, the differences between previously described factors affecting IPV were not compared. Our patients used a twice-daily formulation of tacrolimus. A once-daily formulation could have increased adherence and been associated with low IPV. Therefore, further studies are required to define the impact of once-daily formulations on adherence and IPV.
In conclusion, we were unable to determine a clear relationship between nonadherent behaviors measured through EM or self-report and tacrolimus IPV in stable renal transplant patients. This result is contrary to what has been considered and implies a lack of clear understanding of the mechanism of tacrolimus IPV which may impact long-term transplant outcomes. More advanced study designs are needed to clarify this.
Materials and methods
This study was conducted using the dataset from a previously reported PRIMA (imPRoving adherence to Immunosuppressive therapy by Mobile internet Application) trial26. The PRIMA trial was a prospective RCT evaluating the effectiveness of 6 months of use of the medication manager application in promoting medication adherence in patients at more than 1 year post-transplant (IRB no. 1306-031-496, Clinicaltrials.gov: NCT 01905514). This post hoc analysis was conducted according to the Declaration of Helsinki and approved by the Seoul National University Hospital Institutional Review Board (IRB No. 2007-059-1140). The written informed consent was obtained from each patient, and also from parents and/or legal guardians for minor age group participants.
Study population
The inclusion criteria of the original RCT were patients aged 15 to 70 years on twice-daily tacrolimus (PROGRAF; Astellas Pharma, Tokyo, Japan), who were over 1 year post renal transplantation. Multi-organ transplantation and pregnant recipients were excluded. Of the total 138 patients enrolled in the original RCT, we included patients with the use of more than 5 months of EM and with four or more measured outpatient tacrolimus level values in this post hoc analysis.
The primary outcome of the analysis was the comparison of the IPV and the time in therapeutic range (TTR) between adherent and nonadherent groups. In addition, we reviewed de novo donor-specific antibodies (dnDSA) incidence and estimated glomerular filtration rate (eGFR) changes between the groups.
Adherence measures
Adherence was measured using EM and self-reports. Three parameters (taking, dosing, and timing) were measured using the Medication Event Monitoring System (MEMS)-V TrackCap EM system (Aardex, Ltd., Zug, Switzerland) for 6 months after enrollment. Taking adherence was defined as the percentage of cap removals compared with the number prescribed for the monitoring period. Dosing adherence was defined as the percentage of days that the patient took the prescribed number of doses. Timing adherence was defined as the percentage of correct dosing intervals plus/minus 1 h of prescribed intake timing. MEMS lids recorded the time and date of bottle opening onto a digital chip, and individual data were downloaded by the study pharmacists using a MEMS reader software program during the participants’ scheduled visit. The cutoff of the nonadherent group was < 98% taking adherence and/or at least one drug holiday based on previous studies13.
Self-reported adherence was measured using the Basel Assessment of Adherence to Immunosuppressive Medication Scale (BAASIS) based on a 4-week recall on days 28, 90, and 180. The BAASIS is a questionnaire of four items: taking and timing of medication use, drug holidays, and dose reduction. Patients with a score of 1 or more in any items of the BAASIS were considered nonadherent27.
Tacrolimus variability
The concentration of tacrolimus was measured by high-performance liquid chromatography-tandem mass spectroscopy with a Waters 2795 Alliance HT system (Micromass, Manchester, UK) from whole blood samples drawn just prior to taking the morning dose in outpatient basis28. The accuracy was from 96.0 to 104.0%. The intraday coefficient variation (CV) varied from 5.2 to 9.3%, and the interday CV ranged from 3.6 to 9.6%. The lower limit of quantification for tacrolimus was 0.8 ng/mL.
Tacrolimus doses were adjusted at the discretion of the attending physician during the original study period in order to maintain its’ target concentration between 4 and 6 in recipients. We used tacrolimus concentrations for calculating tacrolimus IPV instead of dose-normalized concentrations. Because dose normalization is a measure of variability of tacrolimus absorption and an indicator for clearance rather than exposure, dose normalization was not used when evaluating tacrolimus IPV8.
The tacrolimus IPV was calculated using formulas based on previous literature29:
Mean absolute deviation (MAD, %) = ([|Xmean − X1| +|Xmean − X2|… +|Xmean − Xn|]/n)/Xmean × 100, where SD is the standard deviation of the tacrolimus concentrations, and Xmean is the mean tacrolimus concentration during the period of adherence measurement.
The TTR was calculated using the Rosendaal method, estimating the number of days within range30.
Statistical analysis
The data are expressed as median ± interquartile range (IQR) or mean ± SD. Comparisons of CV and MAD between groups were analyzed using an independent t test. A correlation analysis (Spearman’s rho) was performed to evaluate the association between IPV and longitudinal adherence or among adherence parameters measured by MEMS.
A two-sided P-value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS (version 21.0 for Windows; IBM Corp., Armonk, NY).
Supplementary Information
Acknowledgements
BAASIS was used under license agreement with the University of Basel, Switzerland.
Abbreviations
- BAASIS
Basal Assessment of Adherence to Immunosuppressive Medication Scale
- CV
Coefficient variation
- CYP
Cytochrome P450
- dnDSA
De novo donor-specific antibodies
- eGFR
Estimated glomerular filtration rate
- EM
Electronic monitoring
- IS
Immunosuppressive
- IPV
Intrapatient variability
- IQR
Interquartile range
- MAD
Mean absolute deviation
- MEMS
Medication event monitoring system
- MMF
Mycophenolate mofetil
- PRIMA
ImPRoving adherence to Immunosuppressive therapy by Mobile internet Application
- RCT
Randomized controlled trial
- TTR
Time in therapeutic range
Author contributions
Concept and design: A.H., S.K.M., J.H., S.M. Analysis and interpretation: H.K., A.H., J.H., S.M. Data collection: H.K., A.H., H.K.K., C.C. Writing the article: H.K., A.H., S.M. Critical revision of the article: A.H., S.K.M., J.H., S.M. Final approval of the article: all. Overall responsibility: S.M.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-84868-5.
References
- 1.Hart A, et al. OPTN/SRTR 2017 annual data report: kidney. Am. J. Transplant. 2019;19(Suppl 2):19–123. doi: 10.1111/ajt.15274. [DOI] [PubMed] [Google Scholar]
- 2.Barbarino JM, Staatz CE, Venkataramanan R, Klein TE, Altman RB. PharmGKB summary: cyclosporine and tacrolimus pathways. Pharmacogenet. Genom. 2013;23:563–585. doi: 10.1097/FPC.0b013e328364db84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77:769–776. doi: 10.1097/01.Tp.0000110408.83054.88. [DOI] [PubMed] [Google Scholar]
- 4.Denhaerynck K, et al. Prevalence, consequences, and determinants of nonadherence in adult renal transplant patients: a literature review. Transpl. Int. 2005;18:1121–1133. doi: 10.1111/j.1432-2277.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 5.Mo H, et al. Association of intrapatient variability of tacrolimus concentration with early deterioration of chronic histologic lesions in kidney transplantation. Transplant. Direct. 2019 doi: 10.1097/txd.0000000000000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanhove T, Vermeulen T, Annaert P, Lerut E, Kuypers DRJ. High intrapatient variability of tacrolimus concentrations predicts accelerated progression of chronic histologic lesions in renal recipients. Am. J. Transplant. 2016;16:2954–2963. doi: 10.1111/ajt.13803. [DOI] [PubMed] [Google Scholar]
- 7.Goodall DL, Willicombe M, McLean AG, Taube D. High intrapatient variability of tacrolimus levels and outpatient clinic nonattendance are associated with inferior outcomes in renal transplant patients. Transplant. Direct. 2017;3:e192. doi: 10.1097/TXD.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzales HM, et al. A comprehensive review of the impact of tacrolimus intrapatient variability on clinical outcomes in kidney transplantation. Am. J. Transplant. 2020 doi: 10.1111/ajt.16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fine RN, et al. Nonadherence consensus conference summary report. Am. J. Transplant. 2009;9:35–41. doi: 10.1111/j.1600-6143.2008.02495.x. [DOI] [PubMed] [Google Scholar]
- 10.Rudd P, et al. Pill count measures of compliance in a drug trial: variability and suitability. Am. J. Hypertens. 1988;1:309–312. doi: 10.1093/ajh/1.3.309. [DOI] [PubMed] [Google Scholar]
- 11.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin. Ther. 1999;21:1074–1090. doi: 10.1016/s0149-2918(99)80026-5. [DOI] [PubMed] [Google Scholar]
- 12.Prendergast MB, Gaston RS. Optimizing medication adherence: an ongoing opportunity to improve outcomes after kidney transplantation. Clin. J. Am. Soc. Nephrol. 2010;5:1305–1311. doi: 10.2215/CJN.07241009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schafer-Keller P, Steiger J, Bock A, Denhaerynck K, De Geest S. Diagnostic accuracy of measurement methods to assess non-adherence to immunosuppressive drugs in kidney transplant recipients. Am. J. Transplant. 2008;8:616–626. doi: 10.1111/j.1600-6143.2007.02127.x. [DOI] [PubMed] [Google Scholar]
- 14.Takemoto SK, et al. A retrospective analysis of immunosuppression compliance, dose reduction and discontinuation in kidney transplant recipients. Am. J. Transplant. 2007;7:2704–2711. doi: 10.1111/j.1600-6143.2007.01966.x. [DOI] [PubMed] [Google Scholar]
- 15.Gokoel SRM, Zwart TC, Moes D, van der Boog PJM, de Fijter JW. No apparent influence of non-adherence on tacrolimus intra-patient variability in stable kidney transplant recipients. Ther. Drug Monit. 2020 doi: 10.1097/FTD.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 16.Leino AD, et al. Assessment of tacrolimus intrapatient variability in stable adherent transplant recipients: Establishing baseline values. Am. J. Transplant. 2018 doi: 10.1111/ajt.15199. [DOI] [PubMed] [Google Scholar]
- 17.Del Bello A, et al. High tacrolimus intra-patient variability is associated with graft rejection, and de novo donor-specific antibodies occurrence after liver transplantation. World J. Gastroenterol. 2018;24:1795–1802. doi: 10.3748/wjg.v24.i16.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodrigo E, et al. Within-patient variability in tacrolimus blood levels predicts kidney graft loss and donor-specific antibody development. Transplantation. 2016;100:2479–2485. doi: 10.1097/TP.0000000000001040. [DOI] [PubMed] [Google Scholar]
- 19.Kimikawa M, Kamoya K, Toma H, Teraoka S. Effective oral administration of tacrolimus in renal transplant recipients. Clin. Transplant. 2001;15:324–329. doi: 10.1034/j.1399-0012.2001.150504.x. [DOI] [PubMed] [Google Scholar]
- 20.Bekersky I, Dressler D, Mekki QA. Effect of low- and high-fat meals on tacrolimus absorption following 5 mg single oral doses to healthy human subjects. J. Clin.. Pharmacol. 2001;41:176–182. doi: 10.1177/00912700122009999. [DOI] [PubMed] [Google Scholar]
- 21.Rancic N, Vavic N, Kovacevic A, Mikov M, Dragojevic-Simic V. Drug-drug interactions of tacrolimus. Hosp. Pharmacol. Int. Multidiscip. J. 2015;2:291–296. doi: 10.5937/hpimj1503291R. [DOI] [Google Scholar]
- 22.Stifft F, Stolk LM, Undre N, van Hooff JP, Christiaans MH. Lower variability in 24-hour exposure during once-daily compared to twice-daily tacrolimus formulation in kidney transplantation. Transplantation. 2014;97:775–780. doi: 10.1097/01.TP.0000437561.31212.0e. [DOI] [PubMed] [Google Scholar]
- 23.Min SI, et al. CYP3A5 *1 allele: impacts on early acute rejection and graft function in tacrolimus-based renal transplant recipients. Transplantation. 2010;90:1394–1400. doi: 10.1097/TP.0b013e3181fa93a4. [DOI] [PubMed] [Google Scholar]
- 24.Lars O, Terrence B. Adherence to medication. N. Engl. J. Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 25.Nevins TE, Robiner WN, Thomas W. Predictive patterns of early medication adherence in renal transplantation. Transplantation. 2014;98:878–884. doi: 10.1097/TP.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han A, et al. Mobile medication manager application to improve adherence with immunosuppressive therapy in renal transplant recipients: a randomized controlled trial. PLoS ONE. 2019;14:e0224595. doi: 10.1371/journal.pone.0224595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barraclough KA, Isbel NM, Johnson DW, Campbell SB, Staatz CE. Once- versus twice-daily tacrolimus: are the formulations truly equivalent? Drugs. 2011;71:1561–1577. doi: 10.2165/11593890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Min SI, et al. Conversion of twice-daily tacrolimus to once-daily tacrolimus formulation in stable pediatric kidney transplant recipients: pharmacokinetics and efficacy. Am. J. Transplant. 2013;13:2191–2197. doi: 10.1111/ajt.12274. [DOI] [PubMed] [Google Scholar]
- 29.Shuker N, van Gelder T, Hesselink DA. Intra-patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant. Rev. (Orlando) 2015;29:78–84. doi: 10.1016/j.trre.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt L, Speckman J, Ansell J. Quality assessment of anticoagulation dose management: comparative evaluation of measures of time-in-therapeutic range. J. Thromb. Thrombolysis. 2003;15:213–216. doi: 10.1023/B:THRO.0000011377.78585.63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.