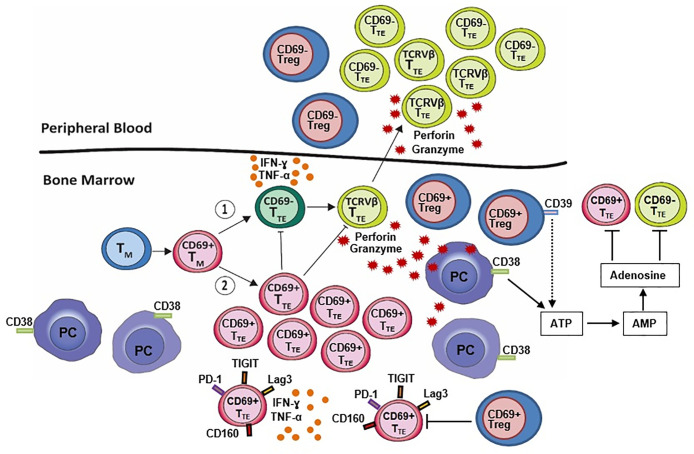
Figure 1.
BM-resident CD69+ regulatory T cells (CD69+ Treg) decrease expression of CD39 which is required for the canonical CD39/CD73 adenosine pathway. This suggests that an alternative adenosine pathway involving ectoenzyme CD38, expressed on malignant plasma cells (PC), is active in the BM niches of NDMM patients. CD8+CD57+ terminal effector T cells (TTE) originate from memory T cells (TM) and may (1) undergo oligoclonal expansion into cytotoxic CD69- TTE (TCRVβ TTE) with high expression of perforin and granzyme B, the ability to eliminate autologous PC in vitro and circulate between BM and PB or, (2) remain within the BM as non-cytotoxic BM-resident CD69+ TTE with high inhibitory checkpoint expression (PD-1, TIGIT, Lag3, CD160) possibly promoted by BM-resident CD69+ Treg. Both CD69- TTE and BM-resident CD69+ TTE produce large amounts of the inflammatory cytokines interferon-γ (IFN-γ) and tumor necrosis factor α (TNF-α). CD69− TTE and CD69+ TTE establish an inverse relationship within the BM-resident T cell compartment of NDMM patients. Changes in Treg and CD69− TTE and CD69+ TTE within the BM-resident T cell compartment of NDMM patients may be critical for myeloma surveillance at the site of disease initiation and progression.