Abstract
LAG-3, through interaction with a variety of ligands regulates T cell function via inhibition of T cell proliferation and activation. It has been demonstrated to be overexpressed on tumor infiltrating lymphocytes (TILs) of a variety of cancers with associated poor outcomes. The purpose of this study is to characterize the expression pattern and clinical significance of LAG-3 in pediatric Hodgkin lymphoma (HL). Patient tumor samples from Children’s Oncology Group clinical trial AHOD0031 with matched patient outcome data were analyzed for the expression of LAG-3 and PD-L1 using immunohistochemistry. 73/115 patients (63%) demonstrated positive LAG-3 staining. No demographic or survival outcome data were significantly associated with LAG-3 expression. Interestingly, patients with the lowest density of expression were found to have the worst EFS, and those with highest density of expression demonstrated the best EFS. There was a positive statistically significant relationship between presence of LAG-3 and PD-L1 expression. This project is innovative in its characterization of LAG-3 as an immune checkpoint target in pediatric HL.
Keywords: pediatric Hodgkin lymphoma, LAG-3, immune checkpoint, immunotherapy
Introduction:
Hodgkin lymphoma is diagnosed in approximately 1,200 children and adolescent patients below 20 years of age annually in the United States. The 5-year overall survival (OS) is 95% with our current risk-stratified, response-based, multi-modality treatment protocols, as well as improvements in supportive care[1]. While approximately 10–30% of patients have primary refractory disease or develop relapse, as many as 70% of those can be salvaged with additional chemotherapy and consolidation using hematopoietic stem cell transplantation. [2–7]. However, there remains a subset of patients who are unable to be cured with existing treatment approaches, as well as those patients who develop late effects including secondary malignancies as a result of conventional chemotherapy, and are in need of innovative therapies.
Over the last decade, novel approaches focus on harnessing the host immune response to eliminate malignant cells. Our normal T cell immune response requires initial activation through the T cell receptor and then a second signal with co-stimulation, as well as balancing of co-inhibition via inhibitory receptors. Tumor cells develop immune escape pathways by up-regulating these inhibitory receptors leading to immune exhaustion, unresponsiveness, and decreased cytotoxic tumor killing[8]. The major immune checkpoints which cancers utilize are members of the B7/CD28 family, to which PD-L1 and CTLA-4 belong. The PD-1/PD-L1 axis has been identified as a key immune escape mechanism in Hodgkin Lymphoma (HL). Amplification of sequences within chromosome 9p24.1 are seen in almost all cases of Hodgkin lymphoma, driving increased PD-L1 expression by the Hodgkin Reed Sternberg cells[9]. This alteration formed the basis for clinical trials which demonstrated the efficacy of PD-1/PD-L1 axis inhibitors in chemotherapy-refractory HL[10]. Use of these agents is still under investigation in pediatric HL, with encouraging interim antitumor results[11,12].
Unfortunately, not all patients respond to these B7 immune checkpoint inhibitors, therefore other co-inhibitory receptors are currently being investigated. Some of these receptors may be advantageous targets but have not yet been evaluated in pediatric HL. Lymphocyte activation gene-3 (LAG-3), one of these co-inhibitory receptors, is expressed on tumor infiltrating lymphocytes (TILs) within a variety of cancers with associated poor outcomes[8,13]. LAG-3 is a member of the immunoglobulin superfamily which shares structural homology with CD4 and binds to MHC II[14,15]. In addition to effector cells, LAG-3 is expressed on activated T and NK cells, T regulatory cells, and plasmacytoid dendritic cells[8]. LAG-3 regulates T cells function via this inhibition of T cell proliferation and activation leading to a state of exhaustion[8]. Its suppressive and immune escape mechanisms are potentiated through the known ligands which include Galectin-3, LSECtin, alpha-synuclein fibers, FGL-1[8,16].
These effects of LAG-3 represent a potential therapeutic pathway for anti-tumor immunity which is currently being evaluated in a variety of adult cancers. However, LAG-3 expression has yet to be evaluated in pediatric cancers, including pediatric HL. The purpose of this study is to explore and characterize the expression pattern and clinical outcome significance of LAG-3 in pediatric HL.
Materials and Methods:
Study Design:
This was a retrospective analysis of previously constructed tissue microarrays (TMA). The primary objectives were to describe LAG-3 expression among children with newly diagnosed HL. In exploratory analyses, we sought to describe any relation between LAG-3 expression and demographic or clinical characteristics, including clinical outcomes.
Patients:
Children’s Oncology Group (COG) study AHOD0031[17] (ClinicalTrials.gov Identifier: NCT00025259), approved by the National Cancer Institute and participating institutional review boards, enrolled patients from September 2002 through July 2009. Eligible patients included those younger than age 22 years with newly diagnosed biopsy-proven intermediate risk HL, defined as AnnArbor stages IB, IAE, IIB, IIAE, IIIA, IVA with or without bulk disease, and IA or IIA with bulk disease. Details of the treatment and outcomes from this study have been published[17].
Tissue microarrays were created using tumor samples collected from 300 subjects who provided informed consent for use of biological specimens for future research. A total of three TMAs were obtained for analyses which has been presented separately[18]. Only two out of the three available TMAs, reflecting 115 unique patient cases, had sufficient remaining material for the evaluation of LAG-3 expression. Demographic data and clinical outcomes for these subjects was extracted by the Children’s Oncology Group from the submitted case report forms.
Immunohistochemistry:
Using previously validated immunohistochemistry techniques[19], paraffin embedded samples were tested for the expression of LAG-3 (Abcam clone 17B4) and PD-L1 (Cell signal E1L3N). Samples were stained for CD30 to better delineate Reed Sternberg cells (RS) from the remainder of the tumor microenvironment. Briefly, TMA slides were baked at 60C for one hour and deparaffinized with xylene and then rehydrated with ethanol and distilled water gradient washes. Antigen unmasking was achieved with citrate unmasking solution and steaming. Slides were incubated with hydrogen peroxide and then TBST/goat serum blocking solution. Slides were incubated with primary antibodies for one hour at room temperature, using 1:500 dilution for PDL1 and 1:200 for LAG-3. Secondary antibody staining was achieved with Equilibrate SignalStain Boost Detection Reagent incubation at room temperature in a humidified chamber for one hour and washed. Signals were generated using 3,3’-diaminobenzidine and counterstained with hematoxylin.
Immune checkpoint staining was compared to positive controls of normal tonsil tissue for LAG-3, and negative controls of 3T3 cells. Expression staining was scored by a Pediatric Pathologist. LAG-3 staining was scored based on percentage of lymphocytes which demonstrated staining, samples were considered positive if >10% of lymphocytes exhibited staining[20]. If 10% of TILs expressed LAG-3 staining those samples were considered as low density of LAG-3 staining, 10–40% considered as moderate staining, and >40% considered as high-density staining. PD-L1 expression threshold of >1% was considered as positive. If multiple cores were stained for the same patient, checkpoint expression was considered positive if at least one core stained positive.
Statistical Analysis:
95% confidence intervals were calculated based on a range of observed prevalence of immune checkpoint expression. Chi-square test was used to assess the correlation between checkpoint expression and both demographic and clinical variables. Survival curves were generated by the Kaplan-Meier method. Event-free survival (EFS), was defined by the time from enrollment on AHOD0031 until treatment failure (disease progression, disease recurrence, biopsy positive residual after completion of all protocol therapy), occurrence of a second malignant neoplasm, or death from any cause[17].
Two-sided P values less than 0.05 were considered statistically significant.
Results:
115 unique HL patient cases with evaluable HL tissue and correlating clinical outcome data were analyzed from 2 TMAs.
The median age in this cohort was 15.8 years (range 3.4–21.0 years). The majority of subjects, 99/155 (86%), had nodular sclerosing subtype. There were 8 (7%) mixed cellularity, 4 (3.5%) lymphocyte predominant and 4 (3.5%) unknown. A majority of the cases, 65/115 (57%) had stage II disease. Among the remaining subjects, there were 8 (7%) with stage I, 21 (18%) stage III, and 21 (18%) stage IV.
Samples from 73 patients (63%) demonstrated positive LAG-3 staining, defined as over 10% of TILs expressing cytoplasmic LAG-3 staining (Figure 1 and Table 1). There was a range of density of staining among the LAG-3 expressing patient cases (Figure 1), 16 (22%) had low density staining, 44 (60%) had moderate staining, and 13 (18%) high density staining. No patient characteristics, including gender, age, or race, were significantly associated with staining density. 71/73 (97%) of patients who expressed LAG-3 were also PDL1+, and 71/106 (67%) of PDL1+ cases were also LAG-3+. Majority of the patients who stained positive for both LAG-3 and PD-L1 (42/71, 60%) displayed moderate LAG-3 expression, with close to a fifth with high LAG-3 expression (13/71). There was a positive statistically significant relationship between presence of LAG-3 and PD-L1 expression (χ2 = 4.24, with 1 degree of freedom, n=73, p=0.04).
Figure 1. IHC Staining.
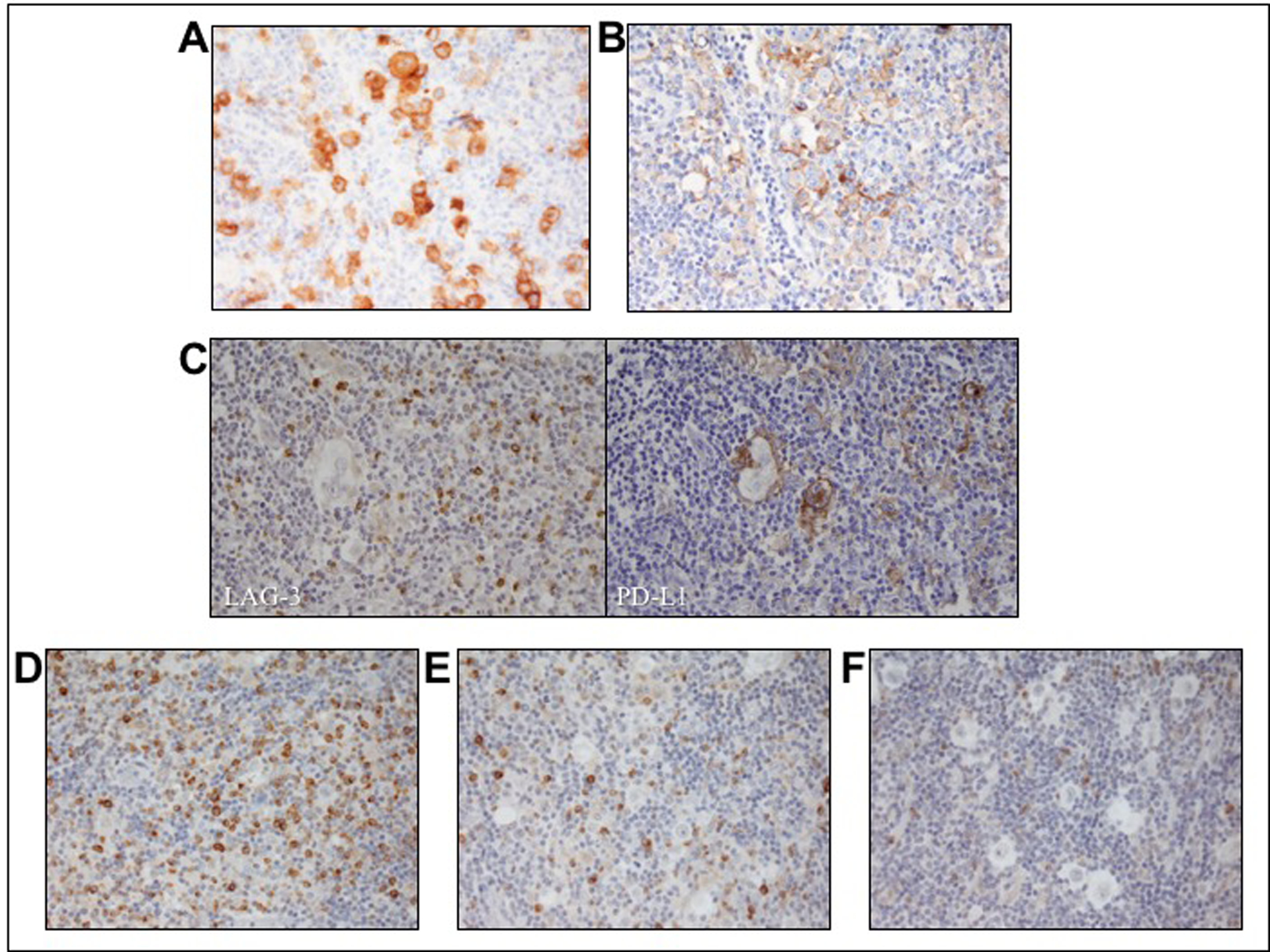
A) CD30 staining Reed-Sternberg Cells B) PD-L1 Staining C) Single patient sample staining positive for both LAG-3 and PD-L1 D) Strong intensity staining with 40% lymphocytes demonstrating cytoplasmic staining E) weak-moderate staining with 15% of positive lymphocytes F) negative for LAG-3 staining with <10% of lymphocytes positive.
Table 1.
Demographics
| LAG-3 Positive (n=73) | LAG-3 Negative (n=42) | ||
|---|---|---|---|
| Male | 41 | 24 | p= 1.0 |
| Female | 32 | 18 | |
| White | 53 | 20 | p= 0.25 |
| Non-white | 20 | 7 | |
| <16 yo | 38 | 25 | p= 0.56 |
| >16 yo | 35 | 17 | |
| Stage I-II | 49 | 24 | p= 0.32 |
| Stage III-IV | 24 | 18 |
None of the available demographic or clinical factors were significantly associated with LAG-3 expression (Table 1 and Figure 2).
Figure 2. Clinical staging breakdown.
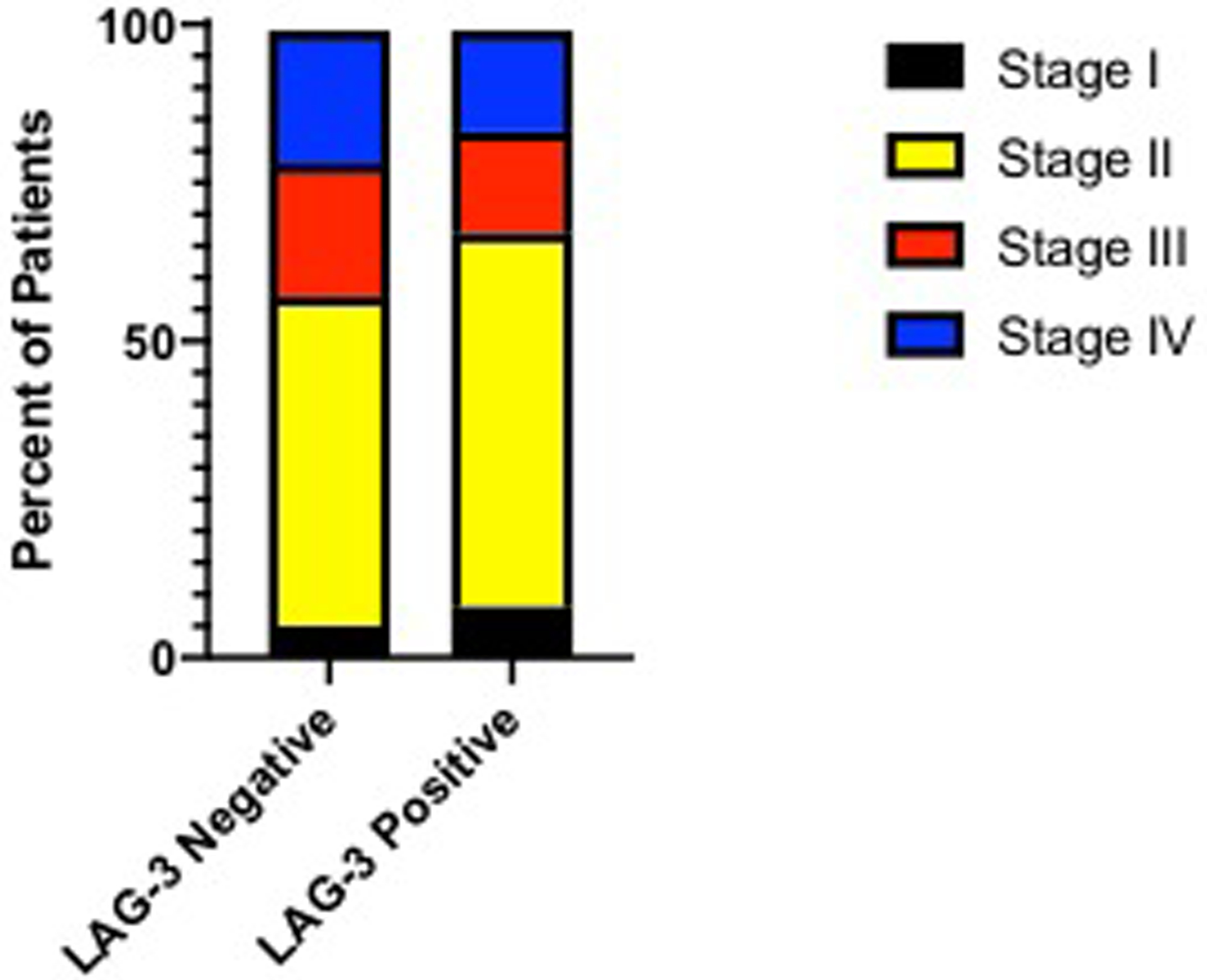
Proportionally there were more stage IV patients in the LAG-3 negative group and more low risk (stage I/II) patients in the LAG-3 positive group.
There were no significant differences in overall survival (OS) nor event free survival (EFS) between LAG-3 positive or negative cases (Figure 3). However, numerically there were more events in the LAG-3 negative group. In terms of degree of LAG-3 expression, patients with lowest positive expression were found to have the worst EFS, and those with highest expression demonstrated the best EFS (Figure 4).
Figure 3. Kaplan Meir Survival Outcomes.
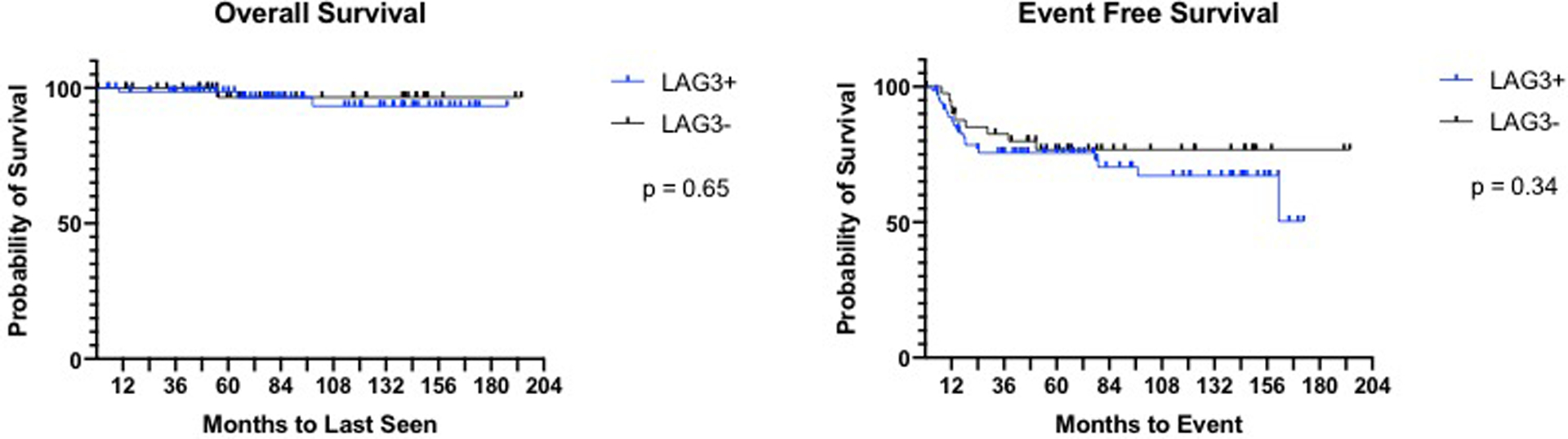
No significant differences in survival outcomes based on LAG-3 expression.
Figure 4. Event Free Survival based on LAG-3 Expression Density.
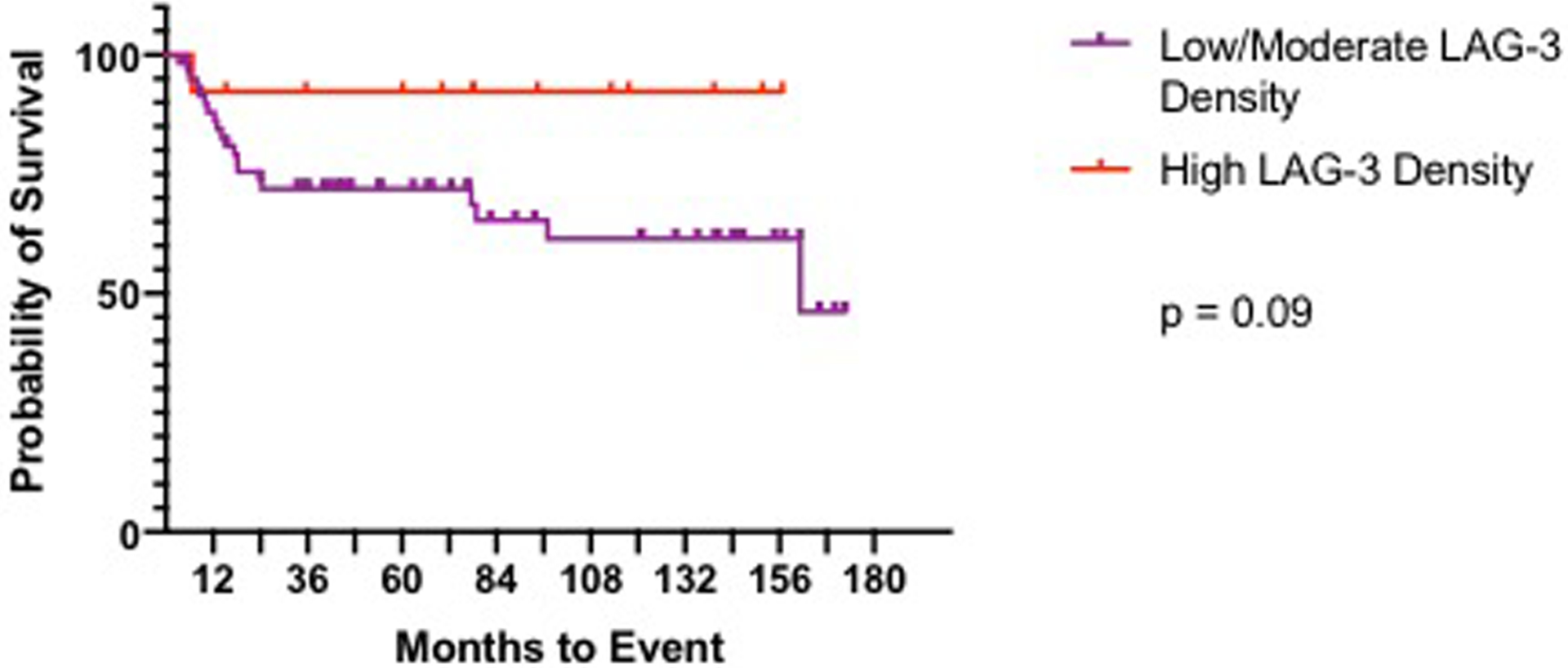
High density LAG-3 expression trended towards improved event free survival compared to low-moderate density staining cases.
Discussion:
HL has a unique tumor microenvironment characterized as an inflamed tumor[21] where less than 10% is comprised of malignant RS cells[22] and the rest is made up of immunosuppressive cell infiltration such as T regulatory cells which block anti-tumor response[8]. Prior studies have evaluated the constellation of suppressive markers in this cellular infiltrate to include FOXP3, TGF-ß, and CTLA-4 [23–25]. In an effort to continue improving response rates, especially for those with advanced, relapsed or refractory disease we need a better understanding of the existing tumor microenvironment and the suppressive methods which malignant cells utilize in order to disrupt tumor immune escape. Here we present data describing the over-expression of an additional suppressive immune checkpoint, LAG-3, in the tumor microenvironment of pediatric HL.
LAG-3 has been evaluated in a variety of adult cancers including ovarian[13] melanoma[8,13,26,27], hepatocellular carcinoma, colon[8,13], colorectal[8,13,16], head and neck squamous cell carcinoma[8,13], chronic lymphocytic leukemia[8,13,28], non-small cell lung cancer (NSCLC)[13,16,29,30], mesothelioma[13], gastric[13,16], soft tissue sarcomas[31], breast[13,16], renal[13], follicular lymphoma[13], prostate[13], anal[13], pancreatic[13], esophageal squamous cell carcinoma[32–34] and pediatric neuroblastoma[16]. Many of these studies have demonstrated that expression of LAG-3 is associated with poor prognostic factors including clinicopathologic characteristics or signs of exhaustion. However, there is a range of reported survival outcomes associated with LAG-3 expression, where it is associated with poor survival in some cancers and favorable prognosis in others[16]. This heterogeneity may be due to differences in patient inclusion criteria, the LAG-3 antibody, staining protocols, and the subjective grading with differing cutoffs to define positivity.
LAG-3 has been previously investigated in two small samples of adult patients with HL [35,36], with discrepant results. However, these studies utilized different techniques including IHC and multiplex immunofluorescence to detect LAG-3 expression. Furthermore, Patel et al describe the low number of T cells expressing LAG-3, whereas el Halibi et al demonstrate the nearly unanimous prevalence of cases which were positive for LAG-3. Even within the same tumor type not all pediatric and adult tumors behave alike nor do they display the same expression patterns, which has been demonstrated by differences in the correlation of gene expression profiling with outcomes in adults and pediatric patients with HL[37]. Even so, we found significant LAG-3 expression similar to the adult studies[13,35], which was not associated with demographics. However, in their subgroup analyses Gandhi et al found that patients with nodular sclerosing HL did not as frequently express LAG-3, as opposed to our cohort which predominantly consisted of patients with nodular sclerosing disease who expressed LAG-3. There was no difference in OS between those who expressed LAG-3 and those who did not. Adult studies describe worse EFS comparing patients with LAG-3+ vs LAG-3- tumors in adult CLL[8], follicular lymphoma[13], head and neck squamous carcinoma[13], NSCLC[13,16], and STS[31]. Although we did not observe a statistically significant association between LAG-3 expression and EFS, there were more events among the patients who were LAG-3 negative than positive (33/42 vs. 21/73). This may relate to a higher proportion of patients who were stage IV among the LAG-3 negative group than among those who were LAG-3 positive (21% vs 16%).
Similar to our cohort, some studies described significant differences in associated prognostic factors and clinical outcomes based on the degree of LAG-3 expression[8,13,16,31,34]. In post-hoc exploration of the different levels of staining density and outcomes we identified a group of LAG-3+ patients with improved ESF. We observed that patients with high density of staining with >40% of lymphocytes staining positive for LAG-3 had improved EFS (Figure 4). HL has been shown to be compartmentalized into distinct immunologic niches[36], which could explain some of this spectrum of expression. This trend in EFS maybe be due to the lack of stage IV patient in this subgroup (High density cohort contained zero stage IV patients vs. 12/60 low density were stage IV). Similarly, a NSCLC study found improved EFS with higher LAG-3 expression compared to low expression in metastatic lymph nodes[30]. One suggested hypothesis for this seemingly contradictory finding of higher expression of an inhibitory checkpoint associated with improved outcomes, is that the high degree of immune inhibition by LAG-3 may lead to negative feedback of inhibitory signals which in turn yields an active immune response in an already inflamed tumor[16]. This is concept has been reported with high expression other immune checkpoints as well which correlate with increased CD8 presence[16], representing active immunity[38] and improved prognosis[39]. Taken together, one could imagine that low level of LAG-3 expression may lead to an exhausted phenotype, but with higher expression the negative feedback kicks in yielding an active tumor response utilizing the already present CD8 TILs. Another possibility for this heterogeneity in outcomes could be due to differential expression of LAG-3 ligands. LAG-3 is known to bind to MHC class II, which is commonly downregulated or absent in HL[40], but also has a number of recently discovered additional binding partners which may disrupt or negate other inhibitory pathways. Of the currently known ligands, only Galectin-3 has been reported to have limited expression in HL[41]. This requires additional investigation in this patient population to correlate LAG-3 ligands with our findings and to better understand these subgroups of expression density with clinical outcomes.
Due to its immunologic role in regulating T cell anti-tumor response, LAG-3 has been evaluated for its activity in conjunction with other immune checkpoints. LAG-3 has been previously described to co-express other inhibitory receptors such as PDL1/PD1 and CTLA4[8,16], which was supported in our cohort with majority of the PDL1+ patients also expressing LAG-3. Currently a number of therapeutic antibodies are under development and investigation, Some believe that anti-LAG-3 could potentially be a more effective therapy due to its effect of both regulatory and effector T cells, as opposed to other checkpoints which do not affect regulatory T cells[13,16]. While anti-LAG-3 showed some benefit in early phase trials, monotherapy only demonstrated limited success[8,13]. Therefore, combinatory regimens with additional checkpoint blockade are being evaluated. In mouse models, the combination of anti-PD-1 and anti-LAG-3 antibodies inhibited tumor growth with enhanced CD8 immune response[13]. These results supported recent clinical trials which have found synergistic effects when combining anti-LAG-3 with anti-PD1 therapy[42–47], and many additional trials are ongoing. In addition to combination therapy, LAG-3 is important in the context of prior immunotherapy as a subsequent line of therapy, as well as a possible biomarker to predict checkpoint response. In NSCLC elevated LAG-3 expression was associated with PD1 axis blockade insensitivity[29] and anti-LAG-3 therapy has been shown to restore lymphocyte tumor reactivity and could help overcome PD-1 axis resistance[26], an area which could be evaluated with a clinical trial utilizing anti-LAG-3 therapy for PD1 axis resistance.
There were some limitations to this study. The clinical trial from which these samples were obtained enrolled patients with intermediate risk HL, therefore it is possible that inclusion of low and higher stage patients might have been able to elucidate more significant relationships between LAG-3 expression and clinicopathologic factors or outcomes. While this cohort contained a sizable number of patients, with the high cure rate in pediatric HL, an even larger cohort might help to detect additional clinical outcome differences between subgroups. Furthermore, this project was not initially powered to detect differences in LAG-3 expression, rather this was part of a larger immune checkpoint investigation. Therefore, future studies would benefit for larger cohorts including relapsed and matched samples to evaluate how immune checkpoint expression changes throughout therapy. Prior studies have demonstrated checkpoint modulation with checkpoint blockade[36,48], therefore it would be of interest to evaluate LAG-3 expression post CTLA4 or PD1 blockade. These studies could evaluate additional checkpoints in the same TME as well as LAG-3 ligands to gain a better understanding of LAG-3 in pediatric HL.
Conclusion:
This project is innovative in its characterization of LAG-3 as an immune checkpoint target present in pediatric HL. These results support further study of whether LAG-3 is a clinically-relevant target for pediatric patients with Hodgkin Lymphoma.
Acknowledgments:
This project was supported by funds from the St. Baldrick’s Foundation and the Children’s Oncology Group Foundation NCTN ITSC-Hematopoietic Malignancies Grant UG1CA233249. Protocol AHOD0031 was supported by National Cancer Institute Grant No. U10 CA98543, NCTN Operations Center Grant U10CA180886 and NCTN Statistics & Data Center Grant U10CA180899 to the Children’s Oncology Group Chair.
Footnotes
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of interest statement: None
References:
- 1.Kelly KM, Hodgson D, Appel B, et al. Children’s Oncology Group’s 2013 blueprint for research: Hodgkin lymphoma. Pediatr Blood Cancer. 2013. June;60(6):972–8. [DOI] [PubMed] [Google Scholar]
- 2.Baker KS, Gordon BG, Gross TG, et al. Autologous hematopoietic stem-cell transplantation for relapsed or refractory Hodgkin’s disease in children and adolescents. J Clin Oncol. 1999. March;17(3):825–31. [DOI] [PubMed] [Google Scholar]
- 3.Shankar A, Hayward J, Kirkwood A, et al. Treatment outcome in children and adolescents with relapsed Hodgkin lymphoma--results of the UK HD3 relapse treatment strategy. Br J Haematol. 2014. May;165(4):534–44. [DOI] [PubMed] [Google Scholar]
- 4.Cole PD, McCarten KM, Pei Q, et al. Brentuximab vedotin with gemcitabine for paediatric and young adult patients with relapsed or refractory Hodgkin’s lymphoma (AHOD1221): a Children’s Oncology Group, multicentre single-arm, phase 1–2 trial. Lancet Oncol. 2018. September;19(9):1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaCasce AS, Bociek RG, Sawas A, et al. Brentuximab vedotin plus bendamustine: a highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood. 2018. July 5;132(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaCasce AS, Bociek RG, Sawas A, et al. Three-year outcomes with brentuximab vedotin plus bendamustine as first salvage therapy in relapsed or refractory Hodgkin lymphoma. Br J Haematol. 2020. February 12. [DOI] [PubMed] [Google Scholar]
- 7.Perales M-A, Ceberio I, Armand P, et al. Role of cytotoxic therapy with hematopoietic cell transplantation in the treatment of Hodgkin lymphoma: guidelines from the American Society for Blood and Marrow Transplantation. Biology of Blood and Marrow Transplantation. 2015;21(6):971–983. [DOI] [PubMed] [Google Scholar]
- 8.Andrews LP, Marciscano AE, Drake CG, et al. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017. March;276(1):80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J Clin Oncol. 2016. August 10;34(23):2690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen R, Zinzani PL, Fanale MA, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J Clin Oncol. 2017. July 1;35(19):2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geoerger B, Kang HJ, Yalon-Oren M, et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): interim analysis of an open-label, single-arm, phase 1–2 trial. The Lancet Oncology. 2020;21(1):121–133. [DOI] [PubMed] [Google Scholar]
- 12.Davis KL, Fox E, Merchant MS, et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1–2 trial. The Lancet Oncology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long L, Zhang X, Chen F, et al. The promising immune checkpoint LAG-3: from tumor microenvironment to cancer immunotherapy. Genes Cancer. 2018. May;9(5–6):176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baixeras E, Huard B, Miossec C, et al. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med. 1992. August 1;176(2):327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huard B, Prigent P, Tournier M, et al. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur J Immunol. 1995. September;25(9):2718–21. [DOI] [PubMed] [Google Scholar]
- 16.Saleh RR, Peinado P, Fuentes-Antras J, et al. Prognostic Value of Lymphocyte-Activation Gene 3 (LAG3) in Cancer: A Meta-Analysis. Front Oncol. 2019;9:1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children’s Oncology Group Study AHOD0031. J Clin Oncol. 2014. November 10;32(32):3651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moerdler SCD, Ewart M, Zang X, Cole P. HHLA2 is a New Immune Checkpoint Expressed in Pediatric Hodgkin Lymphoma. AACR Advances in Pediatric Cancer Research Conference September 2019, Montreal, Canada. 2019. [Google Scholar]
- 19.Janakiram M, Chinai JM, Fineberg S, et al. Expression, Clinical Significance, and Receptor Identification of the Newest B7 Family Member HHLA2 Protein. Clin Cancer Res. 2015. May 15;21(10):2359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen BJ, Dashnamoorthy R, Galera P, et al. The immune checkpoint molecules PD-1, PD-L1, TIM-3 and LAG-3 in diffuse large B-cell lymphoma. Oncotarget. 2019;10(21):2030–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kline J, Godfrey J, Ansell SM. The immune landscape and response to immune checkpoint blockade therapy in lymphoma. Blood, The Journal of the American Society of Hematology. 2020;135(8):523–533. [DOI] [PubMed] [Google Scholar]
- 22.Swerdlow SH. WHO classification of tumours of haematopoietic and lymphoid tissues. International Agency for Research on Cancer; 2017. [Google Scholar]
- 23.Karyampudi L, Lamichhane P, Krempski J, et al. PD-1 Blunts the Function of Ovarian Tumor-Infiltrating Dendritic Cells by Inactivating NF-kappaB. Cancer Res. 2016. January 15;76(2):239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvaro T, Lejeune M, Salvado MT, et al. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005. February 15;11(4):1467–73. [DOI] [PubMed] [Google Scholar]
- 25.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001. August;182:207–14. [DOI] [PubMed] [Google Scholar]
- 26.Taube JM, Young GD, McMiller TL, et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin Cancer Res. 2015. September 01;21(17):3969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gros A, Robbins PF, Yao X, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014. May;124(5):2246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Xiang Y, Ding L, et al. Using gene co-expression network analysis to predict biomarkers for chronic lymphocytic leukemia. BMC Bioinformatics. 2010. October 28;11 Suppl 9:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Datar I, Sanmamed MF, Wang J, et al. Expression Analysis and Significance of PD-1, LAG-3, and TIM-3 in Human Non-Small Cell Lung Cancer Using Spatially Resolved and Multiparametric Single-Cell Analysis. Clin Cancer Res. 2019. August 1;25(15):4663–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hald SM, Rakaee M, Martinez I, et al. LAG-3 in Non-Small-cell Lung Cancer: Expression in Primary Tumors and Metastatic Lymph Nodes Is Associated With Improved Survival. Clin Lung Cancer. 2018. May;19(3):249–259.e2. [DOI] [PubMed] [Google Scholar]
- 31.Que Y, Fang Z, Guan Y, et al. LAG-3 expression on tumor-infiltrating T cells in soft tissue sarcoma correlates with poor survival. Cancer Biol Med. 2019. May;16(2):331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun N, Li Y, He J. [Clinical relevance of common inhibitory immune checkpoint genes in esophageal squamous cell carcinoma]. Zhonghua Yi Xue Za Zhi. 2018. June 5;98(21):1703–1706. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Chen D, Zhao Y, et al. Characterization of LAG-3, CTLA-4, and CD8(+) TIL density and their joint influence on the prognosis of patients with esophageal squamous cell carcinoma. Ann Transl Med. 2019. December;7(23):776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Liu YD, Luo YL, et al. Prognostic Value of Lymphocyte Activation Gene-3 (LAG-3) Expression in Esophageal Squamous Cell Carcinoma. J Cancer. 2018;9(22):4287–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.el Halabi L, Adam J, Marty V, et al. Strong expression of the immune checkpoint regulators LAG3 and Tim3 in hodgkin lymphoma. American Society of Hematology; Washington, DC; 2016. [Google Scholar]
- 36.Patel SS, Weirather JL, Lipschitz M, et al. The microenvironmental niche in classic Hodgkin lymphoma is enriched for CTLA-4-positive T cells that are PD-1-negative. Blood. 2019. December 5;134(23):2059–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metzger ML, Mauz-Korholz C. Epidemiology, outcome, targeted agents and immunotherapy in adolescent and young adult non-Hodgkin and Hodgkin lymphoma. Br J Haematol. 2019. June;185(6):1142–1157. [DOI] [PubMed] [Google Scholar]
- 38.Fumet JD, Richard C, Ledys F, et al. Prognostic and predictive role of CD8 and PD-L1 determination in lung tumor tissue of patients under anti-PD-1 therapy. Br J Cancer. 2018. October;119(8):950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fridman WH, Pagès F, Sautes-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nature Reviews Cancer. 2012;12(4):298–306. [DOI] [PubMed] [Google Scholar]
- 40.Roemer MG, Advani RH, Redd RA, et al. Classical Hodgkin Lymphoma with Reduced beta2M/MHC Class I Expression Is Associated with Inferior Outcome Independent of 9p24.1 Status. Cancer Immunol Res. 2016. November;4(11):910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Haene N, Maris C, Sandras F, et al. The differential expression of Galectin-1 and Galectin-3 in normal lymphoid tissue and non-Hodgkin’s and Hodgkin’s lymphomas. International journal of immunopathology and pharmacology. 2005;18(3):431–443. [DOI] [PubMed] [Google Scholar]
- 42.Ascierto PA, Melero I, Bhatia S, et al. Initial efficacy of anti-lymphocyte activation gene-3 (anti–LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti–PD-1/PD-L1 therapy. American Society of Clinical Oncology; 2017. [Google Scholar]
- 43.Ascierto PA, Bono P, Bhatia S, et al. LBA18Efficacy of BMS-986016, a monoclonal antibody that targets lymphocyte activation gene-3 (LAG-3), in combination with nivolumab in pts with melanoma who progressed during prior anti–PD-1/PD-L1 therapy (mel prior IO) in all-comer and biomarker-enriched populations. Annals of Oncology. 2017;28(suppl_5). [Google Scholar]
- 44.Piha-Paul SA, Amin A, Kovacs C, et al. A phase 2, open-label study of the combination of spartalizumab (PDR001) and LAG525 for patients with advanced solid tumors and hematologic malignancies. American Society of Clinical Oncology; 2018. [Google Scholar]
- 45.Haines BB, Javaid S, Cui L, et al. Blockade of LAG-3 amplifies immune activation signatures and augments curative antitumor responses to anti-PD-1 therapy in immune competent mouse models of cancer. AACR; 2017. [Google Scholar]
- 46.Ghosh S, Sharma G, Travers J, et al. TSR-033, a novel therapeutic antibody targeting LAG-3, enhances T-cell function and the activity of PD-1 blockade in vitro and in vivo. Molecular cancer therapeutics. 2019;18(3):632–641. [DOI] [PubMed] [Google Scholar]
- 47.Savitsky D, Ward R, Riordan C, et al. INCAGN02385 is an antagonist antibody targeting the co-inhibitory receptor LAG-3 for the treatment of human malignancies. AACR; 2018. [Google Scholar]
- 48.Bjoern J, Lyngaa R, Andersen R, et al. Influence of ipilimumab on expanded tumour derived T cells from patients with metastatic melanoma. Oncotarget. 2017;8(16):27062. [DOI] [PMC free article] [PubMed] [Google Scholar]


