Highlights
-
•
Specific pharmacological activation of alternative Ca2+ conductance.
-
•
Activation of TRPV channels, abolishes the rhythmic contractile activity.
-
•
Tocolysis was consistently induced on human myometrial strips.
Abbreviations: BKCa, Ca2+-activated large conducting potassium channels; IbTX, Iberiotoxin; MLC, Myosin light chain; MLCP, Myosin light chain phosphatase; PLC, Phospholipase C; PTB, Preterm birth; TRP, Transient Receptor Potential proteins; TRPV, Transient Receptor Potential of the vanilloid family; USMC, Uterine Smooth Muscle Cells
Keywords: Calcium conductance, Cell signaling, TRPV channels, Tocolytic effect, Uterine contractions
Abstract
Background
Human myometrium is a therapeutic target for labor induction and preterm labor.
Objective
This study aimed to assess the physiological role of alternative calcium conductance on contractions triggered by uterotonic drugs in human myometrium. Membrane conductances, supported by TRPV channels, may provide alternative pathways to control either free intracellular and/or submembrane Ca2+-concentration, which in turn will modulate membrane polarization and contractile responses.
Study design
Uterine biopsies were obtained from consenting women undergoing elective caesarean delivery at term without labor (N = 22). Isometric tension measurements were performed on uterine smooth muscle strips (n = 132). Amplitude, frequency, and area under the curve (AUC) of phasic contractions, as well as resting tone, were measured under various experimental conditions. Immuno histo- and cyto-chemistry, as well as Western blot analyses, have been performed with specific antibodies against TRPV1, TRPV3, and TRPV4 proteins. TRPV4 agonists; GSK1016790A, 4αPDD, and 5,6-EET were used to assess the role of TRPV4 channels on rhythmic activity triggered by 30–300 nM oxytocin. 5 μM of ruthenium red was used as an efficient blocker of ionic current through TRPV4 channels. Nanomolar concentrations of iberiotoxin (IbTX) were also used to confirm the downstream involvement of BKCa channels in controlling uterine reactivity and contractility.
Results
The expression of TRPV3 and TRPV4 isoforms has now been demonstrated in human myometrial tissue and cell culture. Nanomolar concentrations of the TRPV4 agonists, (either GSK1016790A or 4αPDD) abolished the rhythmic contractions, resulting in a rapid and consistent tocolytic effect. While 5 μM of ruthenium reversed this tocolytic effect. The addition of IbTX (a BKCa channel blocker) reversed the effects of GSK1016790A. Carvacrol, a TRPV3 agonist, had similar tocolytic effects on rhythmic contractions albeit at higher concentrations. This inhibitory effect was also reversed by ruthenium red.
Conclusion
Collectively, these data suggest that activation of TRPV4 leads to a Ca2+ entry and subsequent BKCa channel activation (increase in open state probability), which in turn hyperpolarizes the myometrial cell membrane, inactivating L-type Ca2+ channels and efficiently abrogates contractile activity. Consequently, alternative Ca2+ conductance supported by TRPV4 plays a physiological role in the modulation of myometrial reactivity.
1. Introduction
Preterm birth (PTB) is the leading cause of infant morbidity and mortality. Thus, decreasing myometrial excitability and contraction was the rationale to delineate new tocolytics, in order to prolong pregnancy and, more importantly, fetal development [1]. Uterine smooth muscle cells (USMC) contract in response to various uterotonic stimuli, involving the activation of G protein-coupled receptors (GPCR) and downstream effectors [2,3]. These pharmacomechanical responses are waved by membrane polarization, which relies on ion channel activation and conductance changes, as well as opposing GPCRs activity, which control the rate of relaxation [2,4]. For many years, the electrophysiological study of myometrial tissues during active labor has relied on the activation of L-type Ca2+ channels (i.e. nifedipine-sensitive channels) and Ca2+ release from intracellular stores [5]. However, little is known regarding the molecular mechanisms involved in the control of the relative quiescence of the myometrium prior to the onset of active labor. One of the major unresolved questions is how the myometrium controls its own excitability, despite the increased expression in L-type Ca2+ channels, gap junctions, and specific GPCRs, in order to yield fully functional myometrium for active labor. More importantly, could a new class of tocolytics allow the neutralization of preterm labor and PTB, which remain major issues in modern obstetrics? [1,6].
Transient receptor potential (TRP) proteins are a broad family of non-selective cationic channels, are regulated by a wide variety of physical and chemical stimuli [[7], [8], [9]]. Since pharmacological tools were not available, the putative physiological roles of these non-voltage gated ionic channels (mainly TRPV isoforms) have been characterized by genomic analysis. Hence, silencing strategies have been used in various tissues, including smooth muscle cells [10], the results of which suggest that these channels are key regulatory proteins in vascular smooth muscles [9,10]. The pharmacological properties of the TRPV channel sub-family have been partially addressed in the last few years [8]. These studies have led to the generation of various agonists and antagonists, which have been tested on cellular systems, primarily from vascular and neurological tissues [11,12].
In human myometrium, the expression of alternative membrane conductance supported by these channels, as much as their activation or inhibition, may exert adaptive effects during pregnancy and labor. Morphological changes are accompanied by a major change in the electrophysiological properties of uterine smooth muscle cells, as reviewed previously [1,13,14]. As such, focal entry, sarcoplasmic reticulum Ca2+ release, as well as feedback control of membrane potential, could represent relevant factors of functional importance to fine-tune cell membrane potential, myometrial tone and contractile properties [5].
The rationale of the present study was to test whether alternative calcium conductance supported by TRPV channels can modulate the excitability and modify the contractile pattern of myometrial strips, given that mechanical stretch has been shown to regulate TRPV4 expression and calcium entry in arterial myocytes [15]. Using new pharmacological tools, our goal was to ascertain the role of TRPV channels on the electrophysiological properties of native human myometrial smooth muscle tissues collected during elective cesarean sections.
2. Materials and methods
2.1. Subjects and sample collection
All biopsy specimens were obtained from patients over 18 years of age admitted for an elective cesarean section. The study was approved by the institutional Ethics Committee for Research on Human Subjects (protocol 2016−1266) and all volunteers gave written informed consent. The inclusion criteria were: 1) gestational age between 370/7 and 400/7 weeks, and 2) singleton gestation. Exclusion criteria included: 1) spontaneous or oxytocin-induced labor and 2) presence of infections (chorioamnionitis, HIV, genital herpes, hepatitis B and C, or 3 vaginal bleedings during the third trimester). During the cesarean section, immediately after delivery, all myometrium biopsies were excised from the upper lip of the lower uterine segment incision in the midline, as previously described [16,17]. Once collected, all tissue biopsies were transported in physiological salt solution and used within 1 h of collection, unless specified otherwise.
2.2. Isolated organ bath contractile activity recordings
Myometrial strips were mounted for isometric recordings under 2 g of resting tension in an isolated organ bath system as previously described [16,17].
2.3. Immunohistochemistry
Paraffin-embedded myometrial tissue sections were freed from paraffin and rehydrated before immunohistochemical staining was performed according to the standard avidin-biotin immunoperoxidase complex technique using the following antibodies: Anti-TRPV1 (Alomone Labs, Jerusalem, Israel; cat#ACC-030; dilution 1:200), anti-TRPV3 (Abcam, Cambridge, UK; cat #ab231150; dilution 1:200), anti-TRPV4 (Abcam; cat #ab39260 dilution 1:200) or rabbit isotype IgG (Dako; Glostrup Denmark; Cat#X0903) as negative control. Diaminobenzidine (DAB; Sigma Fast kit, Sigma-Aldrich; Oakville ON Canada; cat#D4168) was used for the detection of the labeled proteins, and the sections were counterstained with Harris modified hematoxylin (Fisher Scientific, Cat#SH26−500D). Slides were scanned with a Hamamatsu Nanozoomer 2.0-RS scanner from the Histology and Electron Microscopy platform at the Faculty of Medicine and Health Sciences of the Université de Sherbrooke.
2.4. Western Blotting analysis
Subcellular fractions were prepared from myometrium biopsies followed by SDS–PAGE and Western blot techniques as previously described [16].
2.5. Myometrial cell culture
Following the biopsy dissection, small uterine strips were dissected in order to obtain the primary uterine smooth muscle cells (USMC). Uterine strips were placed into a six-well plate with Dubecco’s eagle media (DMEM F12). This media contained 15 mM HEPES, 1% penicillin and streptomycin, and 5% fetal bovine serum (FBS), all from Wisent Inc. St-Bruno, Quebec). The strips were incubated at 37 °C with 5% CO2. The media was changed every other day. Following 4–5 weeks, after a single passage, USMC were cultured on collagen-coated coverslips in the six-well plate wells, fixed in 4% PFA and fluoro-cytochemistry analyses were performed with the antibodies described above. The secondary antibody was an anti-rabbit IgG coupled to ALEXA fluor 488 (Millipore-Sigma, Oakville, Ontario).
2.6. Drugs and chemical reagents
GSK 1016790 A (TRPV4 agonist), 4αPDD were purchased from Cayman Chemical Company (Ann Arbor, Michigan). GSK 1016790A was dissolved in 100 % EtOH at 1 mM. The final bath concentration of EtOH never exceeded 0.1 %. Ruthenium red (RR) was bought from Honeywell Fluka (Mexico City, Mexico). Oxytocin (from Sigma-Aldrich) and Iberiotoxin (IbTX- from Alomone Lab) were diluted in distilled water, prior to final dilution in Krebs solution. Exogenous activators and inhibitors were added separately to the tissue bath in 30-min intervals. Fresh Krebs solution was prepared daily and used for equilibration, testing and washout periods.
2.7. Data and statistical analysis
The amplitude and area under the curve (AUC) were calculated each recording [16,17] Contractile activities were quantified by calculating the amplitude and the area under the curve for each experimental condition using Sigma Plot 11.0 (SPSS-Science, Chicago, IL). Normal distributions were assessed and analyzed using a paired t-test (parametric test). Non-normally distributed data were analyzed with a Wilcoxon signed-rank test (paired non-parametric test). Differences were considered significant when P < 0.05.
3. Results
3.1. Study population and biopsies
The demographic characteristics of all patients who participated in the study are summarized in Table 1. The study group was comprised of 22 healthy pregnant women (22 Caucasian) with a mean age of 31 ± 5 years with a mean body mass index (BMI) of 26.3 (11 patients were below 25, 2 were between 25 and 30 and 9 above 30). Participants underwent cesarean delivery at 38 [4] ± 02 weeks of gestation, in average. Of the study population, 91 % did not smoke during pregnancy. All biopsy specimens were recovered from the surgery room by the obstetrician in duty and assessed for their functional and pharmacological properties as previously reported [16,17].
Table 1.
Demographic Data of Patients included in the study.
| Characteristics | |
|---|---|
| Number of Patients | 22 |
| Gestational age | Weeks |
| Mean ± SEM | 384 ± 02 |
| BMI | N (%) |
| <25 | 11 (50) |
| 25−30 | 9 (41) |
| >30 | 2 (9) |
| Smoking During Pregnancy | (%) |
| Yes | 9% |
| No | 91 % |
| Total number of Strips | 132 |
Alternative Ca2+ conductance and tocolysis.
3.2. Effect of a TRPV4 agonist on the rhythmic contractile activity
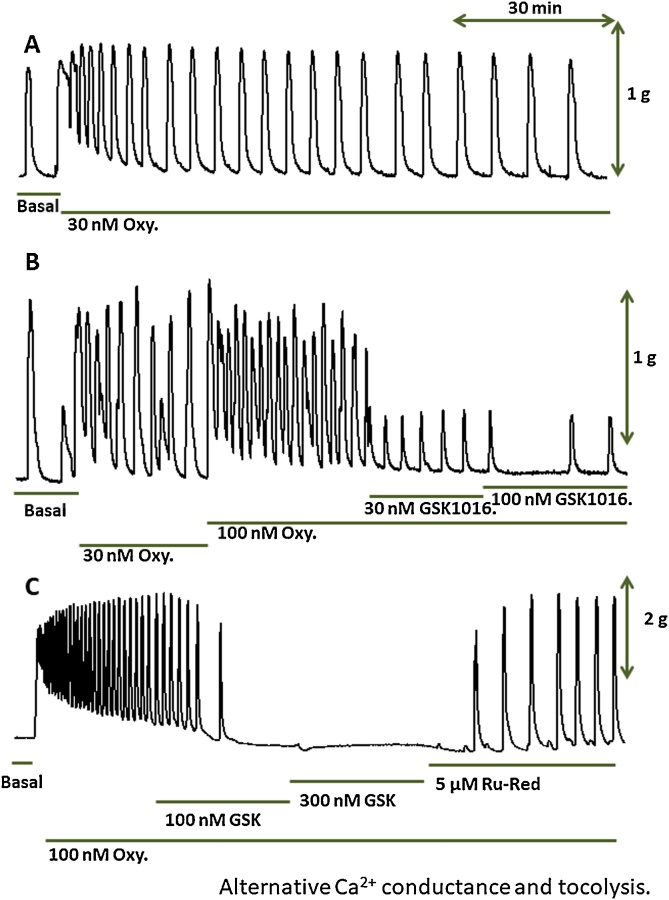
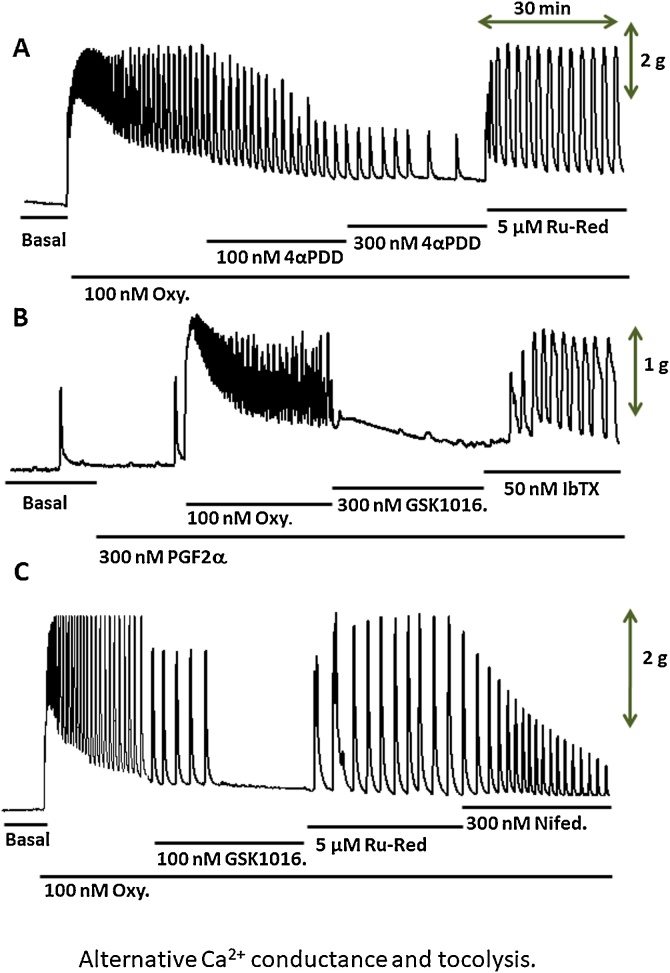
Fig. 1A depicts a typical recording of basal and oxytocin-induced contractile activity [18] from a native human myometrial strip for over a 90-minute period as a time control. Fig. 1B shows the cumulative addition of oxytocin on rhythmic contractions, as well as the concentration-dependent responses to 30 and 100 nM GSK1016790A (GSK1016), which resulted in a consistent tocolytic effect on the same kind of preparation. The third illustration shows the complete tocolytic effect induced by slightly higher concentrations of the GSK 1016790A compound at 100 and 300 nM, respectively. This consistently observed tocolytic effect appears counter-intuitive since the activation of TRPV4 (increased open state probability by the agonist) should induce an inward Ca2+ current. It has been suggested that the tocolytic effect might be explained by the secondary activation of BKCa channels of the surface membrane, as the result of Ca2+ entry through nearby TRPV4 channels [19]. In this specific protocol, ruthenium red was used to block the putative ionic current through TRPV4 channels. The addition of 5 μM ruthenium red, a polyamine compound, known to block ionic currents through TRPV channel proteins, quickly reversed (within 5−8 min) the tocolytic effect induced by the TRPV4 agonist.
Fig. 1.
Effect of GSK1016790A on oxytocin-induced contractions of human myometrial strips.
A: Time control of the effect of 100 nM oxytocin (Oxy) over a 90 min-period. B: Typical recording of a rhythmic contractile activity induced by 100 nM oxytocin, followed by the cumulative addition of 30 and 100 nM GSK1016790A (a TRPV4 agonist). X and Y scale bars represent 30 min and 1 g, respectively. C: The effect of 100 nM GSK1016790A was reversed by the addition of 5 μM Ruthenium Red (a blocker of TRPV4).
Y scale bar =2 g.
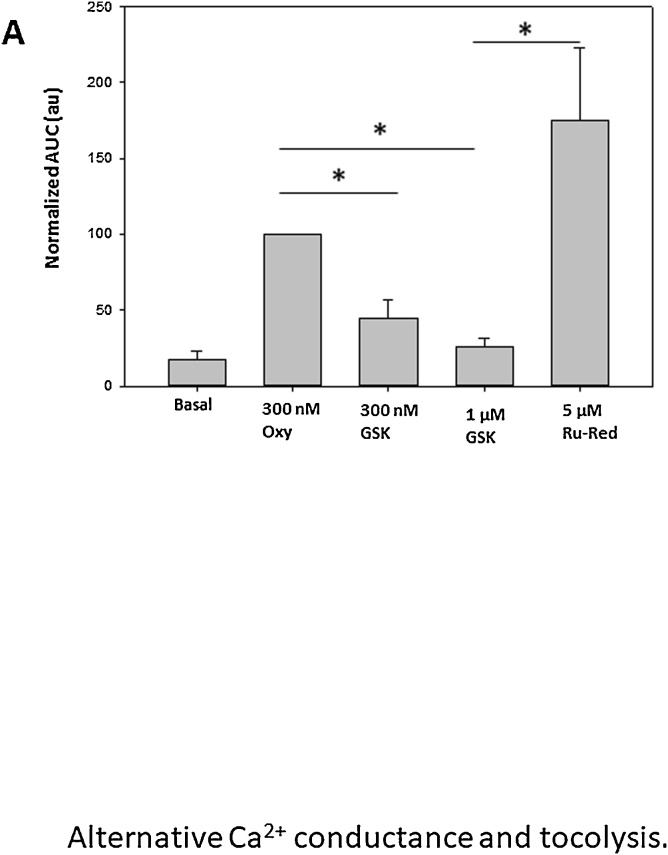
3.3. Quantitative analysis
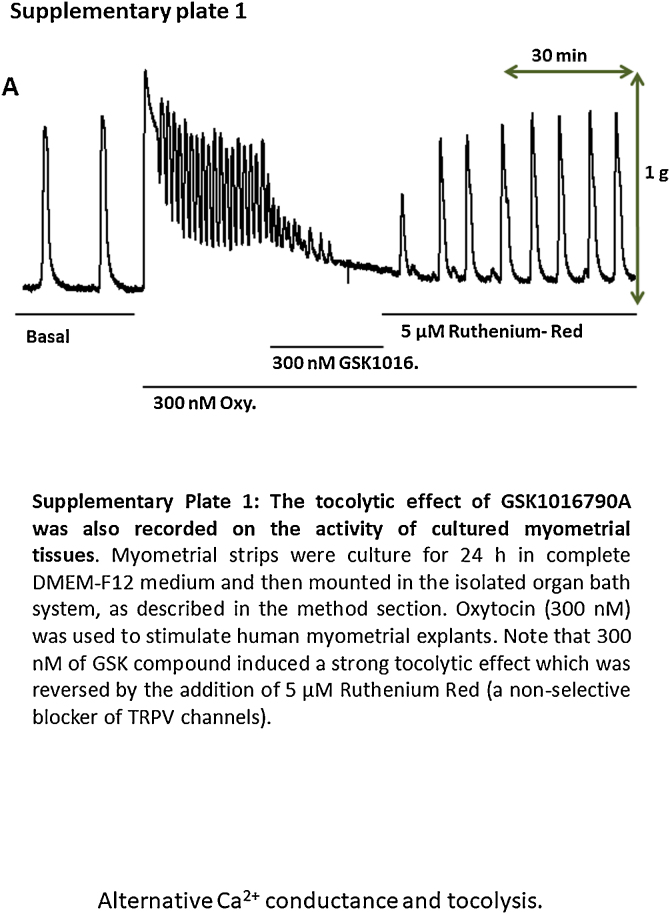
Fig. 2 depicts the mean contractile activity responses under various experimental conditions, normalized to the oxytocin response on the normalized area under the curve (AUC). This effect was highly reproducible (n = 61 strips from 11 independent biopsies) and was consistently reversed by the addition of 5 μM ruthenium red. This statement is also supported by the supplementary data provided as Supplementary Plate 1, where the addition of 100 nM GSK1016790A induced a rapid inhibitory effect of the contractile activity triggered by 300 nM oxytocin. The tocolytic effect of GSK 1016790A was also reversed by ruthenium red (see supplementary material).
Fig. 2.
Quantitative analysis of the effects of GSK on normalized AUC of the oxytocin-induced contractions.
A: Bar histogram represents means ± SEM. The mean SEM value for 300 nM Oxy was 19 % (N = 8). These results demonstrate that GSK1016790A consistently decrease the contractile activity, while 5μM Ruthenium red reversed the tocolytic effect of GSK1016790A.
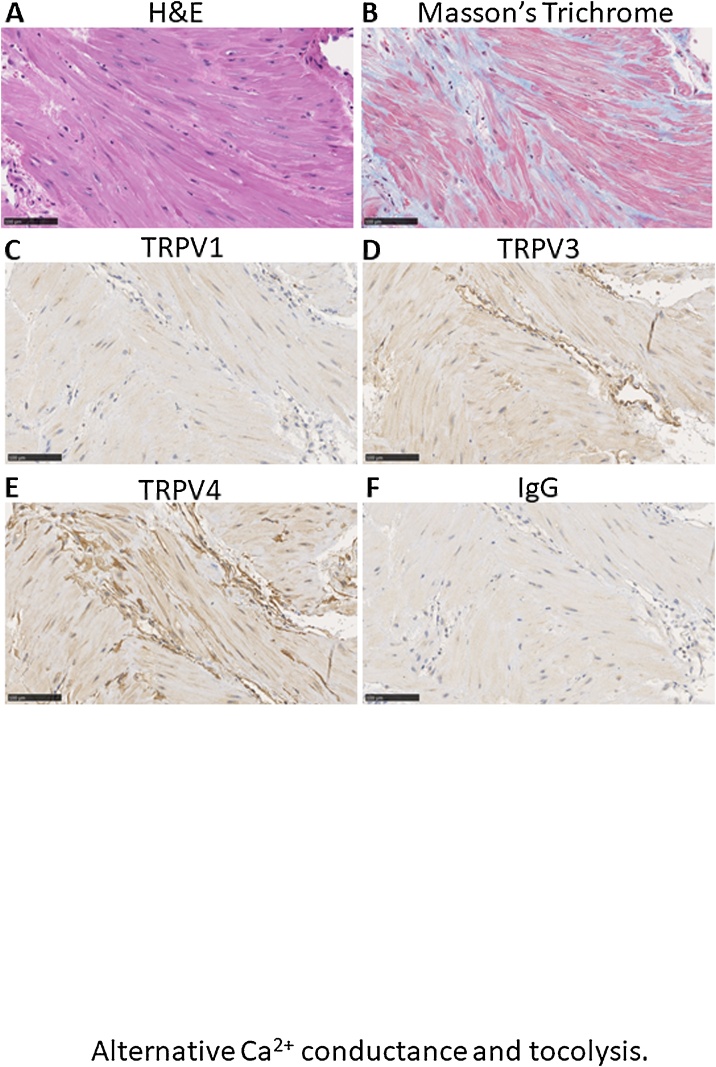
3.4. Detection of TRPV isoforms in native myometrial tissues
Fig. 3(A–B) displays different color staining, following H&E, and Masson’s trichrome performed on native myometrial tissues in order to reveal putative resident inflammatory cells and collagen levels. Moreover, immuno-histochemical analyses were performed using specific antibodies against TRPV1, TRPV3, or TRPV4, using a rabbit non-targeting IgG as negative control (Fig. 3 C–F). These photomicrographs reveal myometrial smooth muscle cell bundles of different orientations, as well as the presence of collagen (Fig. 3B, blue staining). Incubation with either primary TRPV1 antibody or isotypic rabbit IgG shows neither specific nor non-specific staining, respectively. However, anti TRPV3 and antiTRPV4 antibodies both revealed brownish positive staining in native human uterine tissue at term and more specifically around vessels. Thus, we conclude that TRPV3 and TRPV4 channel proteins are present in uterine and vascular smooth muscle cells. The latter has already been assessed and reported [20].
Fig. 3.
Relative expression of TRPV1, TRPV3 and TRPV4 in myometrial tissue.
Representative images from histological staining of native uterine tissue sections using A: hematoxylin & eosin () and B: Masson’s trichrome stains. Immunohistochemistry staining of C: TRPV1, D: TRPV3 and E: TRPV4, compared to F: IgG isotype (negative control) of native uterine tissue sections. Scale bar represents 100 μm.
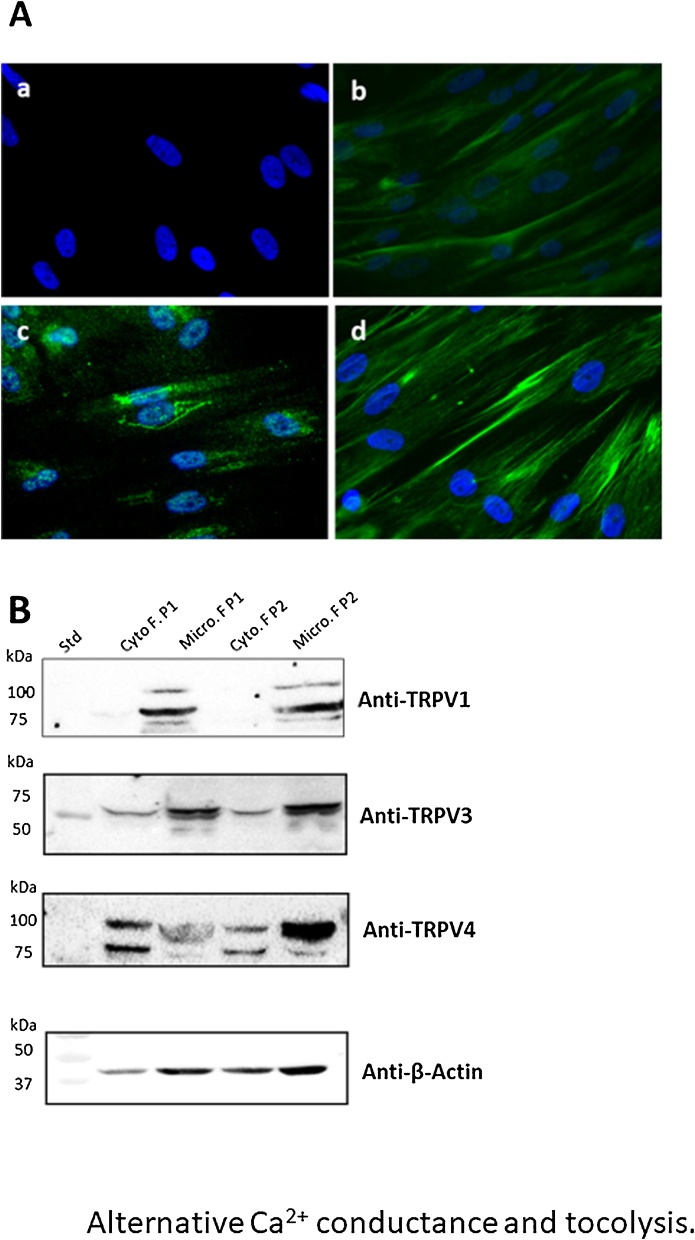
One objective of this work was to delineate the presence, or not, of TRPV isoforms in USMC. For this task, specific commercial antibodies raised against TRPV1, TRPV3, and TRPV4 isoforms have been used on myometrial cell culture. Immuno-cyto fluorescence assays were performed. Fig. 4A shows that, compared to the negative control (absence of primary antibody), the TRPV3 and TRPV4 isoforms were detected in cultured myometrial cells (Fig. 4A), while the immunofluorescence signal to track TRPV1 were faint (Fig. 4A).
Fig. 4.
Immuno-cyto-fluorescence and Western Blot analyses in USMCs and myometrial tissue subcellular fractions.
A: Fluoro-immunocytochemistry detection of TRPV protein isoforms in cell culture derived from human myometrial tissues, a: DAPI staining of the nuclei, b: Anti TRPV1, c: Anti TRPV3, d: Anti-TRPV4 revealed by a secondary antibody coupled to ALEXA fluor 488. B: Detection of three TRPV proteins in the cytosolic and microsomal fractions prepared from human myometrial tissues at term, from two independent patients (P1 and P2). From top to bottom: anti-TRPV1, anti-TRPV3, anti-TRPV4 and anti-β-actin (loading control) immunoblots are shown. Collectively, these immunostainings of the different TRPV isoforms confirmed the presence of the ligand-gated ion channels in myometrial cells and microsomal fractions from five independent preparations of human myometrial tissue.
Complementary Western blot analyses have been performed to assess whether or not those three TRPV isoforms were detected in subcellular fractions prepared from myometrial tissue from independent patients. Fig. 4B shows typical Western blots performed on cytosolic and microsomal fractions prepared from human myometrium tissues at term, using specific antibodies against TRPV1, TRPV3 and TRPV4. Major immuno-reactive bands were observed in the microsomal fractions with all three TRPV antibodies, although some immunostaining was revealed in the cytosolic fractions with the TRPV3 and TRPV4 antibodies. A monoclonal antibody against β actin has been used to assess loading control. Such data confirm the presence of these two TRPV isoforms in the microsomal -membrane- fractions from native and cultured human uterine tissues.
3.5. Effect of two other drugs on oxytocin−induced contractions
Another TRPV4 agonist has been assessed on the contractile activity of human myometrial strips [21]. Fig. 5A shows that sub-micromolar concentrations of 4αPDD induced a consistent tocolytic effect on functional human uterine bundles. This effect was reversed by the addition of ruthenium red. Interestingly, Fig. 5B demonstrate that the tocolytic effect induce by nM concentration of GSK1016790A in the presence of PGF2α and Oxytocin was abolished in the presence of 50 nM IbTX, a highly selective blocker of BKCa channels [17].
Fig. 5.
Tocolytic effects of 4αPDD and GSK1016790A were reversed by ruthenium red and IbTX.
A: Tocolytic effect of 100 and 300 nM 4αPDD on the oxytocin-induced contractile activity. This effect was reversed by 5 μM Ruthenium Red. B: This recording demonstrates that the tocolytic effect of 100 nM GSK1016790A on the uterotonic-induced contractile activity (300 nM PGF2α and 100 nM oxytocin) was reversed by 50 nM IbTX, a specific inhibitor of BKCa channels. C: This recording illustrates the difference of tocolytic mechanism between 100 nM GSK1016790A and 300 nM Nifedipine. The former results in the full inactivation of contractions, while the latter reduced the amplitude of the contractions, likely by blocking the L-type Ca2+ channels.
In this context, it was of interest to compare the tocolytic effect induced by the high-affinity TRPV4 agonist (GSK1016790A) and nifedipine, known as an L-type Ca2+ channel blocker. Fig. 5C elegantly shows the difference in tocolytic kinetics between GSK1016790A and nifedipine on the same human uterine bundle. For this task, we took advantage of the ruthenium red blocking effect on TRPV4 channels. Note that 100 nM GSK1016790A triggered a rapid quiescence of the contractile tissue initially induced by 100 nM oxytocin, while 300 nM nifedipine induced a time-dependent inhibition of the amplitude of the rhythmic contractions.
4. Discussion
4.1. Main findings
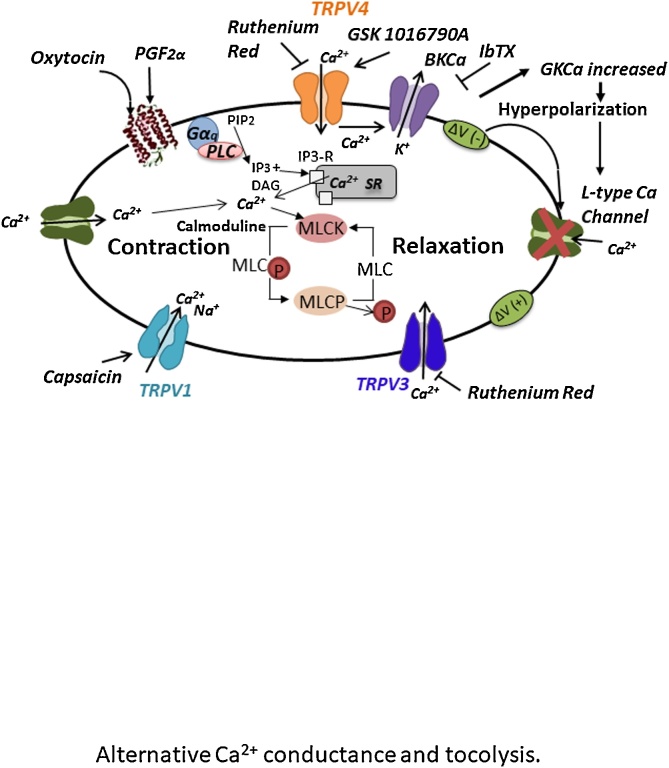
In the present study, we investigated the functional roles of TRPV3 and TRPV4 on human myometrial contractile properties. Our data demonstrate that TRPV4 agonists displayed strong, rapid and reversible tocolytic activity on human myometrial tissue. This effect is likely due to the secondary activation of GKCa conductance, supported by BKCa channels, as suggested by previous reports [8,17]. These current results add to previous findings describing the utero-relaxing actions of BKCa channels in vitro [24,25]. Hence, ruthenium red, a non-specific blocker of non-selective channels, appeared as an efficient tool to demonstrate reversibility induced by the activation of TRPV channels in this functional context. This complementary set of data suggested a putative role for a new class of tocolytic drugs, which would be able to decrease the reactivity of human myometrium through the pharmacological activation of TRPV4 channels and the downstream activation of BKCa channels [21] as illustrated on the graphic summary (Fig. 6).
Fig. 6.
Graphic summary: Involvement of TRPV4 and BKCa activation results in a strong tocolytic effect on human myometrial strips.
The TRPV4 agonist (GSK1016790A) may exert a tocolytic effect on human myometrial strips by activating the BKCa channels. This strong inhibitory effect is likely due to a localized Ca2+ entry, which in turn activates BKCa channels. An increase in open state probability of BKCa channels results in membrane hyperpolarization and concomitant inactivation of Nifedipine-sensitive Ca2+ channels, while either ruthenium red (a TRPV4 blocker), or iberiotoxin (IbTX) -a high-affinity BKCa blocker- reverses this tocolytic effect [17].
4.2. Clinical relevance
The pharmacological activation of TRPV channels in myometrial cells would appear to generate a localized Ca2+ influx, susceptible of activating BKCa channels, resulting in turn in a hyperpolarization of the membrane potential as well as a concomitant inactivation of nifedipine-sensitive Ca2+ channels and the abrogation of phasic contractions, thereby potentially facilitating the management of preterm labor. However, further studies are warrant of the current TRPV4 agonists as potential pharmacological drugs of clinical interest.
4.3. TRPV4 agonist as an unlikely tocolytic compound
Oxytocin was shown to enhance the amplitude and frequency of phasic contractions in the nanomolar range [3,18]. However, in the present study, there was no coordinated activation between oxytocin and TRPV4 agonist. Our current view is that these TRPV agonists likely activate an inward Ca2+ flux, as previously demonstrated on vascular smooth muscle cells [8,25], which in turn would directly or indirectly activate an outward K+ current through BKCa channels, located nearby. Activation of this specific K+ conductance is responsible for membrane hyperpolarization and concomitant Ca2+ channel inactivation resulting in contractile quiescence. The specific activation of this efficient transmembrane process may ultimately silence uterine contractions, which would be of key clinical interest in preterm labor [4,24,26]. We have already demonstrated that HC-067047, a TRPV4 antagonist, had only a slight effect on oxytocin-induced contractile activity [17]. In contrast, ruthenium red, a polyamine compound, was reported to be a potent inhibitor of the current passing through TRPV4 and TRPV3 channels [[21], [22], [23]]. We are now reporting that this compound was able to reverse the effect TRPV4 agonists, both GSK1016790A and 4αPDD compounds (Figs. 1, 2 and 5). This relevant observation proves thatTRPV4 channels were functionally involved in the induced tocolytic responses and that the uterine tissues were still responsive following these pharmacological challenges [27].
During vaginal delivery, thus under physiological condition, there is no direct evidence of TRPV4 and BKCa channel activation. That would be an issue to assess in vivo, in animal models. However, during parturition under physiological conditions, the activation of BKCa channels is likely under the control of sub-membrane Ca2+ concentrations, phosphorylation levels and membrane-voltage as previously demonstrated [4,24,25]. The pharmacological activation of TRPV4 by selective agonists could, therefore, represent a novel strategy to enhance the K+ conductance of USMC plasma membranes. It has furthermore been demonstrated that activation of K+ channels and specifically of BKCa channels can hyperpolarize myometrial smooth muscle cells [24], thus in turn inactivating the Ca2+ current through L- type Ca2+ channels. To support this functional hypothesis, the addition of 50 nM Iberiotoxin (IbTX), a high-affinity BKCa channel blocker with a low Kd of 0.5 nM, subsequently to the tocolytic effect of the drugs, allowed the recovery of contractile activity [17]. Our current results confirm the presence and strengthen the functional role for TRPV4 channels in gravid murine and human uterine tissues, as previously suggested [17,28]. However our data, obtained on human myometrium, are at odds with a previous report stating that pharmacological TRPV4 activation in mouse uterine tissues, enhanced the tone of the uterus [29]. This is not what we have consistently observed and quantified in human myometrium bundles, by several investigators in our group [17].
5. Conclusions
Collectively, this study demonstrates that the pharmacological activation of non-selective membrane conductance could be used to inhibit uterine contractions. Assessment of the functional involvements of these cationic conductances, permeable to Ca2+ and now delineated in USMC, reveal that TRPV channel agonists represent a consistent, albeit indirect, tocolytic agents at least in vitro. A rapid switch from phasic contractions to quiescence by this new class of tocolytics may potentially be of clinical interest in delaying parturition in preterm labor.
Ethical statements
The study was approved by the institutional Ethics Committee for Research on Human Subjects (protocol 2016−1266) and all volunteers gave written informed consent.
This study was financially supported by the FMHS, Université de Sherbrooke and Natural Sciences and Engineering Research Council of Canada (NSERC) grant. DV received a summer studentship from the Faculty of medicine and health sciences, and a studentship from the Star Foundation from Québec.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
The authors wish to thank all of the obstetrical staff from the Department of Obstetrics and Gynecology at the ‘’Centre Hospitalier Universitaire de Sherbrooke’’ (CHUS), who participated in the sample collection procedure. O.G. was a recipient of an MD summer-studentship from the Faculty of Medicine and Health Sciences (FMHS), Université de Sherbrooke.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eurox.2021.100124.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Groom K.M., Bennett P.R. Tocolysis for the treatment of preterm labour - a clinically based review. Obstet Gynaecol. 2004;6(1):4–11. doi: 10.1576/toag.6.1.4.26973. [DOI] [Google Scholar]
- 2.Parkington H.C., Tonta M.A., Davies N.K., Brennecke S.P., Coleman H.A. Hyperpolarization and slowing of the rate of contraction in human uterus in pregnancy by prostaglandins E2 and F2α: involvement of the Na+pump. J Physiol. 1999;514(Pt 1):229–243. doi: 10.1111/j.1469-7793.1999.229af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janicek F., Franova S., Nosalova G., Visnovsky J. In vitro contractile response of human myometrium to oxytocin, PGF2α, bradykinin and ET-1. Bratisl Lek Listy. 2007;108:174–178. [PubMed] [Google Scholar]
- 4.Zhou X.B., Wang G.X., Ruth P., Huneke B., Korth M. BK(Ca) channel activation by membrane-associated cGMP kinase may contribute to uterine quiescence in pregnancy. Am J Physiol Cell. 2000;279:C1751–C1759. doi: 10.1152/ajpcell.2000.279.6.C1751. [DOI] [PubMed] [Google Scholar]
- 5.Wray S., Jones K., Kupittayanant S., Li Y., Matthew A., Monir-Bishty E. Calcium signaling and uterine contractility. J Soc Gynecol Investig. 2003;10:252–264. doi: 10.1016/s1071-5576(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 6.Clark S.L., Belfort M.A., Dildy G.A., Herbst M.A., Meyers J.A., Hankins G.D. Maternal death in the 21st century: causes, prevention, and relationship to cesarean delivery. Am J Obstet Gynecol. 2008;199(1) doi: 10.1016/j.ajog.2008.03.007. 36.e1-5. [DOI] [PubMed] [Google Scholar]
- 7.Cloutier M., Campbell S., Basora N., Proteau S., Payet M.D., Rousseau E. 20-HETE inotropic effects involve the activation of a nonselective cationic current in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2003;285(3):L560–L568. doi: 10.1152/ajplung.00381.2002. [DOI] [PubMed] [Google Scholar]
- 8.Earley S. Transient receptor potential channels in the vasculature. Physiol Rev. 2015;95(2):645–690. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H. TRP channel classification. Adv Exp Med Biol. 2017;976:1–8. doi: 10.1007/978-94-024-1088-4_1. [DOI] [PubMed] [Google Scholar]
- 10.Godin N., Rousseau E. TRPC6 silencing in primary airway smooth muscle cells inhibits protein expression without affecting OAG-induced calcium entry. Mol Cell Biochem. 2007;296:193–201. doi: 10.1007/s11010-006-9309-1. [DOI] [PubMed] [Google Scholar]
- 11.Earley S., Heppner T.J., Nelson M.T., Brayden J.E. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 12.Miehe S., P. Schmidt T., Löhn M., Kleemann H.-W., Licher T., Dittrich W. Inhibition of diacylglycerol-sensitive TRPC channels by synthetic and natural steroids. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbara M.S. Hormonal signaling and signal pathway crosstalk in the control of myometrial calcium dynamics. Semin Cell Dev Biol. 2007;18(3):305–314. doi: 10.1016/j.semcdb.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith R. Parturition. N Engl J Med. 2007;356:271–283. doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- 15.Dalrymple A., Mahn K., Poston L., Songu-Mize E., Tribe R.M. Mechanical stretch regulates TRPC expression and calcium entry in human myometrial smooth muscle cells. Mol Hum Reprod. 2007;13(3):171–179. doi: 10.1093/molehr/gal110. [DOI] [PubMed] [Google Scholar]
- 16.Corriveau S., Rousseau E., Berthiaume M., Pasquier J.C. Lipoxygenase and cyclooxygenase inhibitors reveal a complementary role of arachidonic acid derivatives in pregnant human myometrium. Am J Obstet Gynecol. 2010;203(3):266–271. doi: 10.1016/j.ajog.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Rousseau E., Labelle K., Massenavette L. Involvement of alternative calcium conductance on human myometrial contractile and pharmacological properties. Curr Opin Gynecol Obstet. 2018;1(1):100–110. [Google Scholar]
- 18.Kawamata M., Tunomura Y., Kimura T., Sugimoto Y., Yanagisawa T.Nishimori K. Oxytocin-induced phasic and tonic contractions are modulated by the contractile machinery rather than the quantity of oxytocin receptor. Am J Pysiol Endocrinol Metab. 2007;292:E992–999. doi: 10.1152/ajpendo.00492.2006. [DOI] [PubMed] [Google Scholar]
- 19.Ying L., Becard M., Lyell D., Han X., Shortliffe L., Husted C.I. The transient receptor potential vanilloid 4 channel modulates uterine tone during pregnancy. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aad0376. [DOI] [PubMed] [Google Scholar]
- 20.Tajada S., Moreno C.M., O’Dwyer S., Woods S., Sato D., Navedo M.F. Distance constraints on activation of TRPV4 channels by AKAP150-bound PKCα in arterial myocytes. J Gen Physiol. 2017;149(6):639–659. doi: 10.1085/jgp.201611709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorneloe K.S., Sulpizio A.C., Lin Z., Figueroa D.J., Clouse A.K., McCafferty G.P. N-((1S)-1-{[4-((2S)-2-{[(2,4dichlorophenyl) sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther. 2008;326(2):432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- 22.Pires P.W., Sullivan M.N., Pritchard H.A., Robinson J.J., Earley S., Pires P.W. Unitary TRPV3 channel Ca2+ influx events elicit endothelium-dependent dilation of cerebral parenchymal arterioles. Am J Physiol Heart Circ Physiol. 2015;309(12) doi: 10.1152/ajpheart.00140.2015. H2031-H241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy T.V., Kanagarajah A., Toemoe S., Bertrand P.P., Grayson T.H., Britton F.C. TRPV3 expression and vasodilator function in isolated uterine radial arteries from non-pregnant and pregnant rats. Vascul Pharmacol. 2016;83:66–77. doi: 10.1016/j.vph.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Moynihan A.T., Smith T.J., Morrison J.J. The relaxant effect of nifedipine in human uterine smooth muscle and the BK(Ca) channel. Am J Obstet Gynecol. 2008;198:237.e1–237.e8. doi: 10.1016/j.ajog.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 25.Naik J.S., Walker B.R. Endothelial-dependent dilation following chronic hypoxia involves TRPV4-mediated activation of endothelial BK channels. Pflugers Archiv. 2018;470(4):633–648. doi: 10.1007/s00424-018-2112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doheny H.C., Lynch C.M., Smith T.J., Morrison J.J. Functional coupling of beta3-adrenoceptors and large conductance calcium-activated potassium channels in human uterine myocytes. J Clin Endocrinol Metab. 2005;90(10):5786–5796. doi: 10.1210/jc.2005-0574. [DOI] [PubMed] [Google Scholar]
- 27.Sedwick C. TRPV4 in the battle of the sexes. J Gen Physiol. 2017;149(6):611. doi: 10.1085/jgp.201711820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vishakha S., Mahendra R., Kannan K., Ramasamy T., Soumen C., Jeevan R.D. Molecular and functional characterization of TRPV4 channels in pregnant and non-pregnant mouse uterus. Life Sci. 2015;122:51–58. doi: 10.1016/j.lfs.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Ying L., Becard M., Lyell D., Han H., Shortliffe L., Husted C.I. The transient receptor potential vanilloid 4 channel modulates uterine tone during pregnancy. Sci Transl Med. 2015;7:319. doi: 10.1126/scitranslmed.aad0376. [DOI] [PubMed] [Google Scholar]