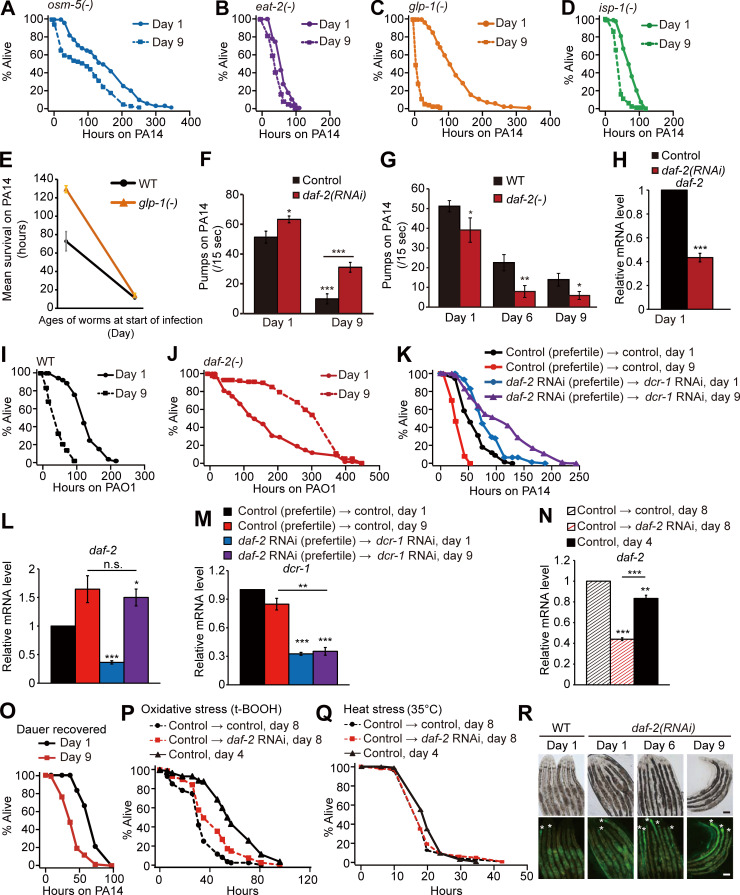
Figure S1.
Age-dependent survival changes of longevity mutants on PA14 and pharyngeal pumping rates and PA14 accumulation in animals with genetic inhibition of daf-2. (A–D) Shown are survival curves of osm-5(p813) [osm-5(−)] (A), eat-2(ad1116) [eat-2(−)] (B), glp-1(e2141) [glp-1(−)] (C), and isp-1(qm150) [isp-1(−)] (D) mutant animals transferred from E. coli OP50 to PA14 at days 1 and 9 of adulthood. (E) Mean survival of WT and glp-1(−) mutant animals transferred from OP50 to PA14 at days 1 and 9 of adulthood. See Materials and methods for immune aging assays using temperature-sensitive glp-1(−) mutants. At least two independent survival assays were performed. (F) Pharyngeal pumping rates of adult WT control RNAi–treated (control) animals transferred from control or daf-2 RNAi plates to PA14 at days 1 and 9 of adulthood (n = 20 from two trials; *, P < 0.05; **, P < 0.01; ***, P < 0.001). (G) Pharyngeal pumping rates of day 1, 6, and 9 adult WT and daf-2(e1370) [daf-2(−)] animals on PA14 (n = 21 from two trials for the pharyngeal pumping rates of WT at day 1; n ≥ 11 from one trial for the pharyngeal pumping rates of WT at day 6 and day 9, n = 12 from one trial for the pharyngeal pumping rates of daf-2(−) animals; *, P < 0.05; **, P < 0.01). (H) qRT-PCR analysis indicates that daf-2 RNAi significantly decreased the mRNA level of daf-2 (n = 3). Error bars represent SEM (two-tailed Student’s t test: ***, P < 0.001). pmp-3 was used as a normalization control. (I and J) Survival curves of WT (I) and daf-2(−) (J) worms transferred from E. coli to P. aeruginosa (PAO1) at days 1 and day 9 of adulthood. (K) Survival curves of day 1 and day 9 WT adult worms treated with daf-2 RNAi during development. We treated worms with daf-2 RNAi during development and subsequently blocked the RNAi effect in young adults by using RNAi targeting dcr-1/DICER1, an essential ribonuclease for RNAi (Dillin et al., 2002; Durieux et al., 2011). (L and M) Changes in the mRNA levels of daf-2 (L) and dcr-1 (M) at days 1 and 9 of adulthood (n = 3). Treatment with daf-2 RNAi during development decreased the mRNA level of daf-2 at day 1, but not at day 9, of adulthood; this is consistent with previous reports showing that knockdown of dcr-1 decreases the effects of daf-2 RNAi in a time-dependent manner (Dillin et al., 2002; Durieux et al., 2011). Error bars represent SEM (two-tailed Student’s t test: *, P <0.05; **, P < 0.01; ***, P < 0.001). ama-1 and pmp-3 were used as normalization controls. (N) Changes in the mRNA level of daf-2 by treatment with daf-2 RNAi from days 4 to 8 of adulthood. The temporal daf-2 RNAi significantly deceased the mRNA level of daf-2 (n = 3). Error bars represent SEM (two-tailed Student’s t test: **, P < 0.01; ***, P < 0.001). pmp-3 was used as a normalization control. (O) Adult worms recovered from dauer exhibited age-dependent decreases in their survival on PA14. One very interesting observation that we made in this study was that daf-2 RNAi during development increased immunocompetence in old adults, but experiencing a dauer stage, which reduces insulin/IGF-1 signaling (IIS; Fielenbach and Antebi, 2008), was not sufficient for delaying immune aging. We speculate that daf-2 RNAi treatment during development may at least partially retain the activity of DAF-16 and HSF-1 during aging for delaying immune aging. In contrast, worms that experience dauer stage may need to completely inhibit DAF-16 and HSF-1 for dauer exit to reach adulthood, leading to normal immunosenescence. Therefore, we speculate that the level of IIS in adult worms that experience dauer stage is similar to that in control adult worms. It will be important to experimentally test this possibility in future studies. (P) Worms treated with daf-2 RNAi from days 4 to 8 of adulthood survived longer under oxidative stress conditions than day 8 control RNAi–treated worms but survived shorter than day 4 control worms. (Q) daf-2 RNAi treatment from middle age (day 4 adulthood) did not increase heat stress resistance in old (day 8 adulthood) worms. (R) Shown are representative images of worms that were pre-treated with daf-2 RNAi, after PA14-GFP exposure for 100 h. Asterisk (*) indicates PA14-GFP (scale bars, 100 μm; magnification, 100×). See Fig. 1 J for semiquantification of PA14-GFP levels shown in R. PA14 colonizes the C. elegans intestinal lumen, and exposure to PA14 ceases pharyngeal pumping in C. elegans (Tan et al., 1999). We wondered whether the reduction of pharyngeal pumping with age caused increased pathogen resistance in daf-2(−) mutants. Although animals with reduced daf-2 functions displayed age-dependent decreases in pharyngeal pumping after infection with the pathogen (Fig. S1, F and G), several lines of evidence indicate that reduced pharyngeal pumping is not the main cause of the increased immunocompetence observed in animals with genetically inhibited daf-2 during aging. First, aged WT worms displayed a reduced pumping rate (Fig. S1, F and G) but exhibited increased susceptibility to PA14 (Fig. 1, A and B). Second, eat-2(−) mutants with defective pharyngeal pumping displayed an age-dependent reduction in pathogen resistance (Fig. 1 A and Fig. S1 B). Third, daf-2 RNAi treatment significantly increased pharyngeal pumping (Fig. S1 F), and nevertheless enhanced survival upon PA14 infection compared with WT worms (Fig. 1, D and F). Fourth, daf-2 RNAi treatment in postreproductive, middle-aged (day 4), WT animals was sufficient to enhance immunocompetence at day 8 adulthood (Fig. 1 H). In addition, although daf-2(RNAi) worms displayed age-dependent increases in pathogen load (Fig. 1 J and Fig. S1 R), old daf-2(RNAi) animals survived longer on PA14 than young daf-2(RNAi) animals did (Fig. 1, D and F). Overall, these data suggest that inhibition of daf-2 can delay immune aging without causing defects in feeding rates, through mechanisms involving resistance against pathogen infection. See Table S1 for additional repeats and statistics for survival data shown in this figure. A previous report suggests that daf-2 mutations extend lifespan but increase the period of frailty in old age by measuring motility and resistance against abiotic stresses, such as heat and oxidative stresses (Bansal et al., 2015). In contrast, we previously reported that daf-2 mutations prolong healthy periods throughout adulthood by measuring maximum physical ability (Hahm et al., 2015). In the current work, we showed that relatively old day 9 daf-2 mutant adults exhibited enhanced immunity, indicating that genetic inhibition of daf-2 can enhance resistance against pathogenic bacteria (biotic stress) in old age. Another previous report demonstrated that daf-2 mutations confer resistance to the colonization by dietary bacteria, E. coli, in the digestive tract (Podshivalova et al., 2017). Here, we found that mutations in daf-2 prevented colonization by pathogenic PA14, but daf-2 RNAi did not. Despite this difference, both daf-2 mutations and daf-2 RNAi enhanced immunocompetence in old age. Thus, enhanced pathogen resistance caused by genetic inhibition of daf-2 does not seem to result from the elimination of the pathogen PA14. In conclusion, genetic inhibition of daf-2 appears to increase at least one aspect of healthspan, resistance against pathogens, by increasing innate immunity in old worms. t-BOOH, tert-butyl hydroperoxide.