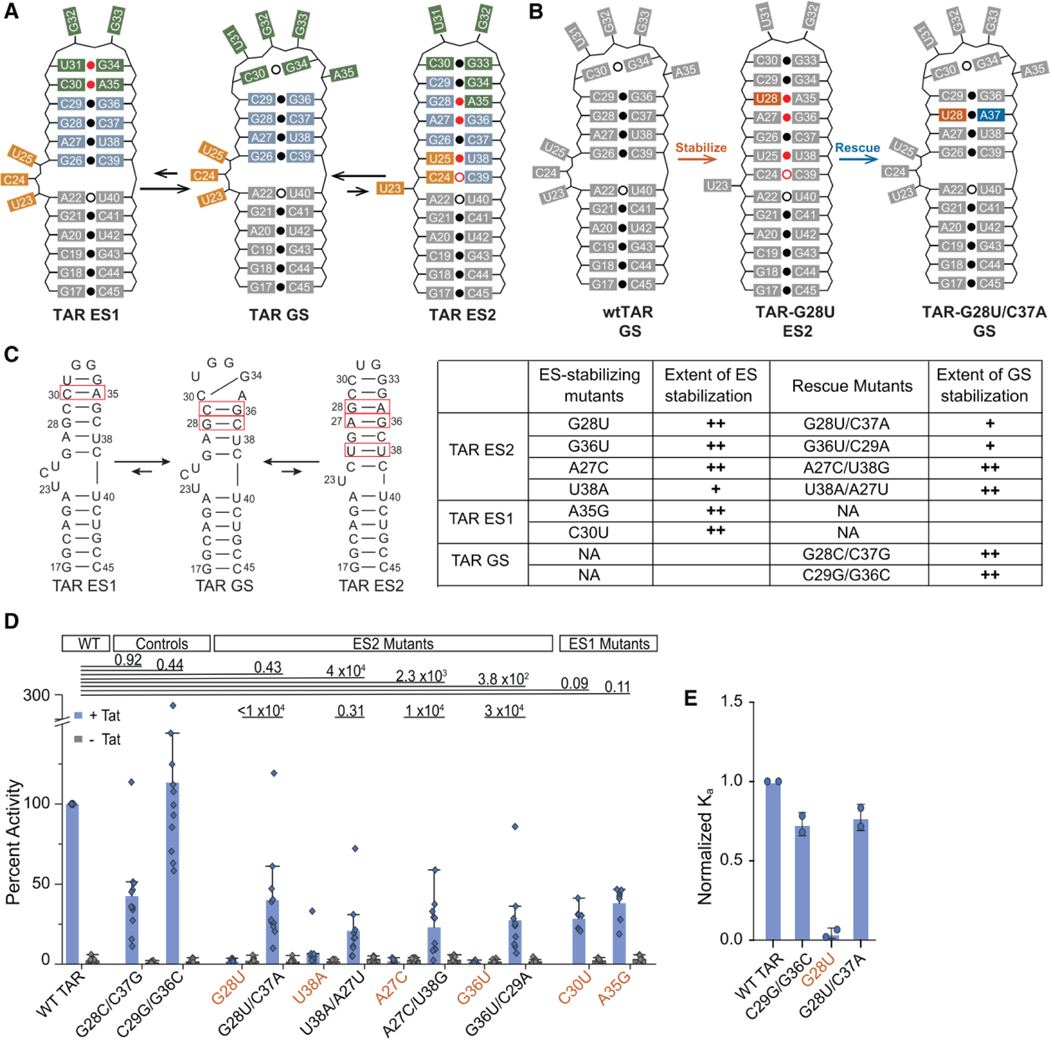
Figure 2.
Probing the conformational equilibrium of HIV-1 TAR in vitro and in vivo. (A) HIV-1 TAR RNA has two ESs that alter base pairing in the bulge (orange), upper stem (blue), and apical loop (green). (B) Example of the stabilize-and-rescue approach for trapping TAR ES2 using the ES-stabilizing mutant G28U (orange) and its corresponding rescue mutation C37A (blue). For A and B, weak base pairs (open dot) and non-Watson-Crick base pairs (red dot) are indicated. (C) Summary of TAR mutants with mutated base pairs highlighted (red box). The extent of stabilization was estimated based on NMR line broadening (Figures S2–S3). (++) minimal line broadening, (+) partial line broadening, and (−) extensive line broadening. (D) Tat-dependent trans-activation assay for TAR mutants determined to be primarily in the GS conformation (black labels) or ES conformation (orange labels) based on NMR. HeLa cells were transfected with pFLuc-TAR reporter plasmid and an RLuc internal control in the presence (+ Tat) or absence (- Tat) of a Tat expression plasmid. Reported values are the quotient of FLuc and RLuc activities with values normalized to WT TAR for every independent replicate reported as the mean ± SD (n=8). Statistical significance of the difference in +Tat and -Tat activity between wtTAR and each mutant was determined after log-transformation of the data and prior to normalization to WT TAR; P-values between TAR variants are shown (two-sided ANOVA). (E) In vitro binding assay between TAR mutants and a peptide of the Tat arginine rich motif. Reported values are the mean ± SD (n=2) of the Ka (1/Kd) values normalized to wtTAR.