Figure 1.
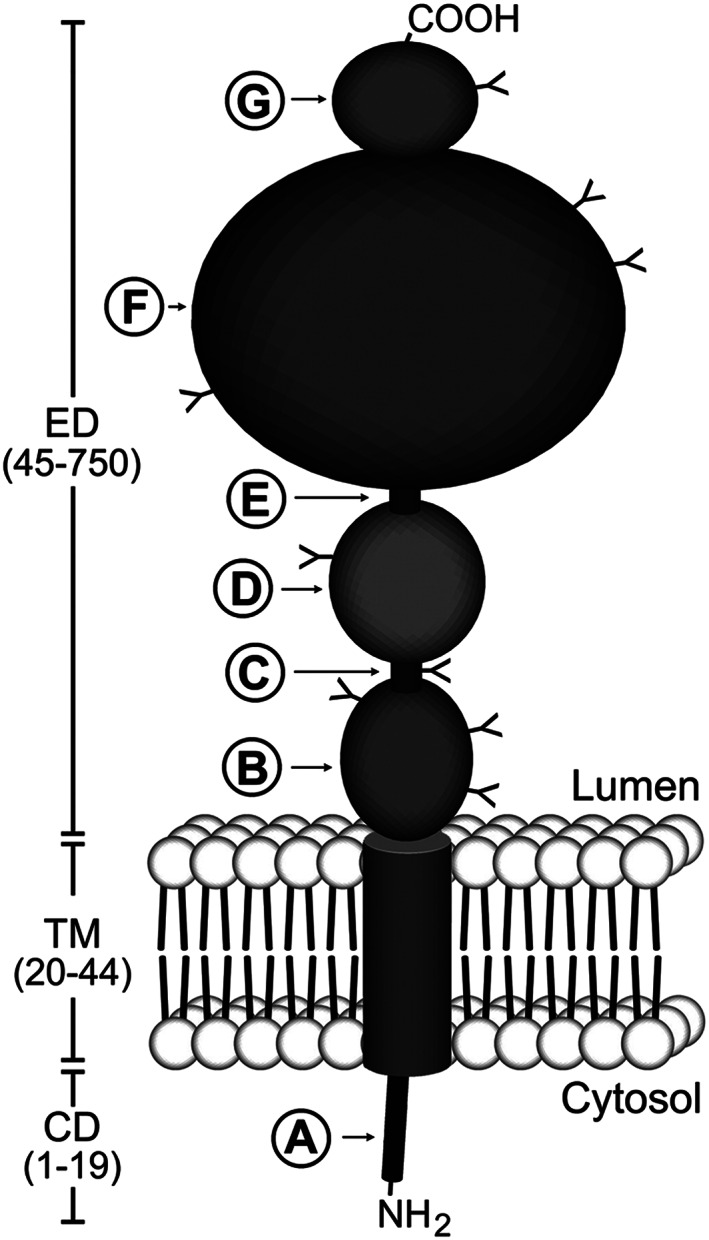
Schematic diagram of prostate‐specific membrane antigen (PSMA) structure. PSMA is a type II transmembrane protein with a short NH2‐terminal cytoplasmic domain (CD), a hydrophobic transmembrane region (TM), and a large extracellular domain (ED). The CD contains an endocytic targeting motif and filamin A (FLNa) binding site (A). The large ED is highly glycosylated with nine predicted N‐glycosylation sites (Y). The ED contains two domains of unknown function that span amino acid residues 44‐150 (B) and 151‐274 (D), proline‐ and glycine‐rich regions that span amino acid residues 145‐172 and 249‐273, respectively (C and E), a catalytic domain that spans amino acid residues 274‐587 (F), and a final domain of unknown function (amino acids 587‐750) to which a helical dimerization domain (amino acids 601‐750) is localized (G). Reproduced with permission from Rajasekaran et al32
