Figure 3. Phosphomimetic S166E mutation in ASF1A promotes histone ubiquitination by facilitating MDC1 phosphorylation by ATM and MDC1-RNF8 interaction at DSBs.
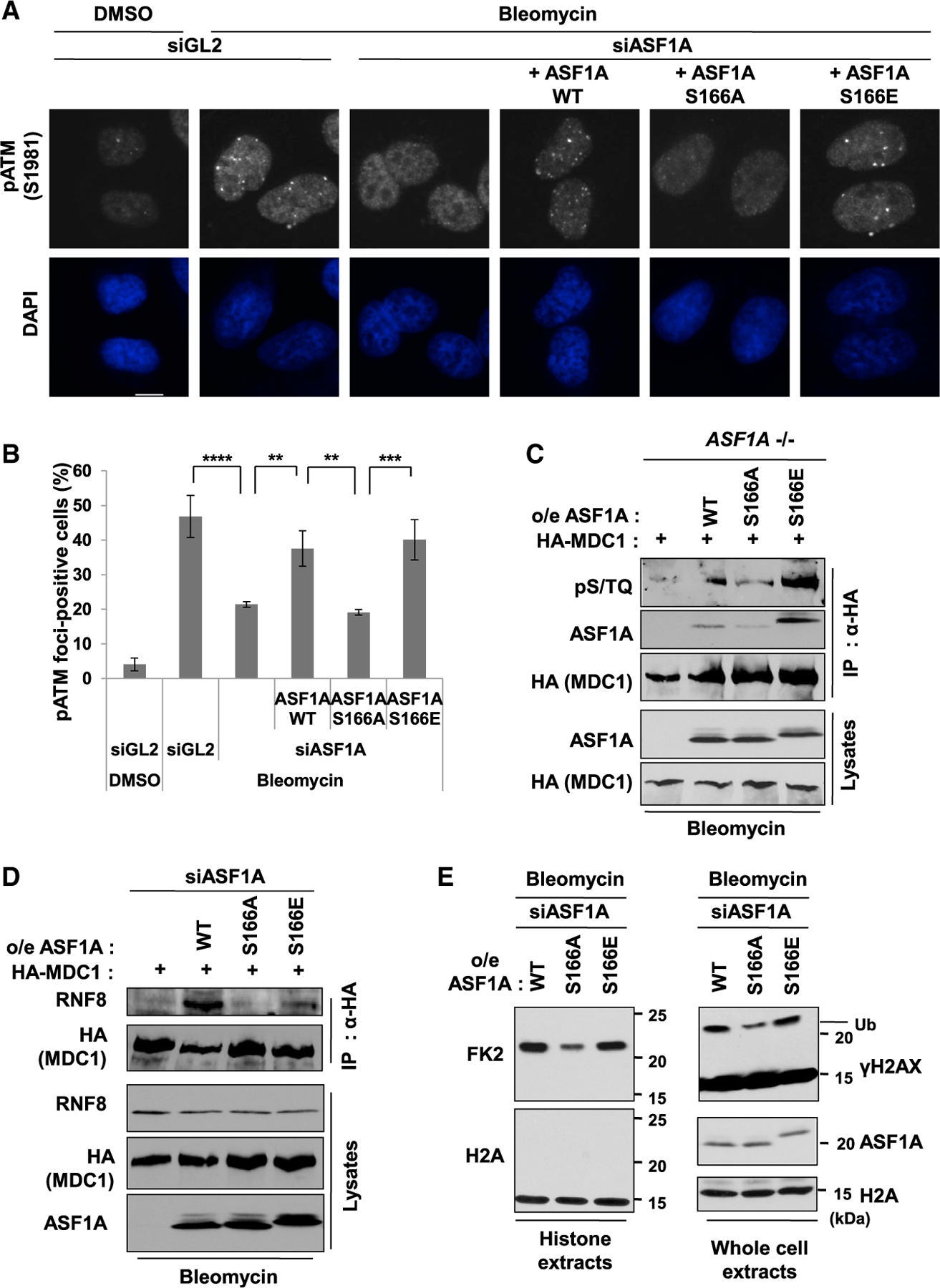
(A and B) DSB localization of phosphorylated ATM at S1981 (pS1981) promoted in presence of phosphomimetic mutation of S166 of ASF1A. Representative images (A) and quantification of cells with more than 5 foci (B). Mean ± SD of triplicates. One-way ANOVA (Tukey’s post hoc test); **p < 0.01; ***p < 0.001; ****p < 0.0001. Scale bar, 10 µm.
(C) S166E mutation of ASF1A permits ATM phosphorylation of MDC1. The ASF1A knockout 293B cells stably expressing WT or S166 mutants were transfected by HA tagged MDC1 plasmids and treated with 20 µg/mL bleomycin treatment for 2 h before harvest. Shown are immunoblots of immunoprecipitates using anti-HA antibody and lysates.
(D) S166E mutation of ASF1A promotes the MDC1-RNF8 interaction. HEK293T cells stably expressing siRNA-resistant WT or S166 mutants were transfected with HA-tagged MDC1 and siASF1A.
(E) Histone ubiquitination promoted by S166E mutation of ASF1A after DSBs. siASF1A-trans-fected U2OS stable cells with ectopic expression of siRNA-resistant WT or S166 mutants were collected, and those histone extracts (left) or whole cell extracts (right) were immunoblotted by indicated antibodies. H2A immunoblots were loading controls for both.
