ABSTRACT
This case report discusses recrudescence of imported Plasmodium falciparum malaria, in the presence of P. falciparum Kelch13 (PfK13) propeller mutation, in a patient diagnosed and fully treated with artemether–lumefantrine under direct observation in Sri Lanka. This patient presented with a history of 5 days of fever following his arrival from the Democratic Republic of Congo (DRC). He had visited Rwanda 1 week before arrival to Sri Lanka. Treatment was commenced with artemisinin-based combination therapy, artemether–lumefantrine, which is the first-line drug recommended for uncomplicated falciparum malaria. Blood smears were negative for parasites by the third day of treatment. Approximately 2 weeks later, he developed fever again and was diagnosed as having a recrudescence of falciparum malaria. He was treated and responded to the second-line antimalarial dihydroartemisinin–piperaquine. Molecular testing of blood taken from the first infection revealed the presence of amino acid substitutions K189T and R561H within the PfK13 gene. R561H mutation is associated with delayed parasite clearance in Southeast Asia. Although seldom reported from DRC, an emergence and clonal expansion of parasites harboring R561H allele has been reported from Rwanda recently; thus, it is likely that this patient may have got the infection from Rwanda. Sri Lanka eliminated malaria in 2016. However, in the backdrop of continuing imported malaria cases, early diagnosis and prompt treatment is essential to prevent the re-establishment of the disease.
INTRODUCTION
The WHO has recommended artemisinin-based combination therapy (ACT) for uncomplicated Plasmodium falciparum malaria and chloroquine-resistant Plasmodium vivax malaria.1 Artemisinin resistance is defined as delayed clearance after therapy (bloodstream parasites are not cleared within 3 days)2 associated with mutations in the propeller domain of the Plasmodium falciparum Kelch 13 (PfK13) gene.3,4 This report from Sri Lanka discusses the genotypic confirmation of PfK13 propeller mutation in a patient diagnosed with imported P. falciparum malaria who presented with late treatment failure.
CASE PRESENTATION
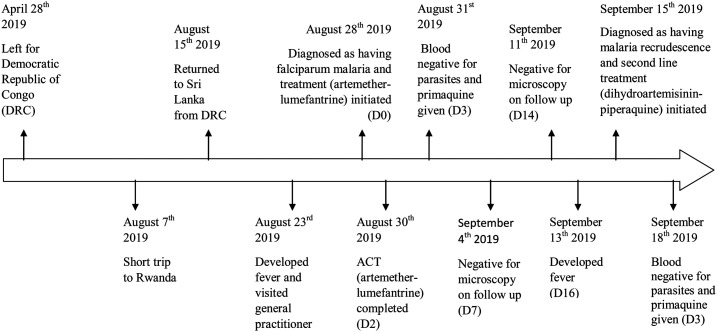
A previously healthy, 41-year-old male, a software developer by profession and a resident of the Western Province of Sri Lanka, returned on August 15, 2019, after a 3-month stay in the Democratic Republic of Congo (DRC). He had visited the city Masaka in neighboring Rwanda 1 week before his arrival to Sri Lanka. He had not taken malaria chemoprophylaxis throughout his stay in Africa; nor had he used any methods to prevent mosquito bites. He developed fever on August 23 and sought medical treatment on the same day. As his fever did not subside, he visited two other general practitioners, who managed the patient as a suspected case of dengue fever, without eliciting travel history. Because of persisting fever, he got admitted to a Teaching Hospital in Colombo on August 28. On examination, the patient was febrile (38°C). Systemic examination was normal; there were no cardiovascular, respiratory, neurological, or gastrointestinal abnormalities, and there was no evidence of hepatosplenomegaly. The hemoglobin was 14.4 g/dL. The platelet count was 40,000/µL, but the dengue NS1 antigen test was negative. Based on the travel history, the patient was further investigated for malaria. Direct microscopy revealed ring stages of P. falciparum with a parasite density of 84,400/µL. The rapid diagnostic test (CareStart Malaria HRP2/pLDH Combo, Access Bio, Inc., Somerset, NJ) was positive for HRP2. Filter paper blood for polymerase chain reaction (PCR) was also taken per routine. While in hospital, he was treated with ACT (artemether 80 mg + lumefantrine 480 mg), the first-line treatment for uncomplicated falciparum malaria in Sri Lanka, by directly observed treatment strategy. Artemisinin-based combination therapy was given twice daily for 3 days after a fatty meal.5 The parasitemia reduced to 1,760 parasites/µL on the next day (D1) and 640 parasites/µL on D2, when the treatment was completed. The blood smear was negative for parasites on D3, and a single dose of primaquine at 0.75 mg/kg was given to clear gametocytes.5 The patient was discharged on clinical recovery and was followed up. The follow-up microscopy on D7 and D14 were negative.
On September 13 (16 days after the initiation of antimalarial therapy), he developed fever again and presented 2 days later where a repeat blood smear revealed the presence of ring stages of P. falciparum parasites with a density of 13,200/µL. A filter paper blood sample was obtained for molecular analysis. Because there was no subsequent visit to a malaria-endemic nation and as he did not respond to the first-line treatment, this case was classified as a probable recrudescence of imported P. falciparum infection. On the same day, the second-line ACT (dihydroartemisinin 120 mg + piperaquine 960 mg) was given in-ward daily for 3 days.1 The patient recovered uneventfully. The blood smears were negative on D3 and remained negative on further follow-up visits on D7, D14, D21, D28, and D42.6
Molecular analysis of the parasite.
The filter paper blood samples taken during the first and subsequent malaria infections were sent to the Environmental Health Institute, Singapore. P. falciparum DNA was extracted from these samples by using DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany). The near-complete PfK13 gene (1950 bp) was amplified by a modified PCR protocol.7 Conventional PCR reactions were performed in 20-μL reactions, by using 1X Phusion Flash master mix (ThermoFisher, Waltham, MA), 0.5 μM of each primer, and 2 μL of DNA template. The oligonucleotide sequences and thermal profile used for the amplification are given in Table 1. Because the DNA was not successfully amplified in the second sample, only the first sample was subject to sequencing.
Table 1.
Oligonucleotides and protocol used for the semi-nested amplification of Plasmodium falciparum Kelch 13 gene
| PCR round | Oligonucleotide name | Oligonucleotide sequence (5′ → 3′) | Amplification protocol | ||
|---|---|---|---|---|---|
| Temperature (°C) | Duration (second) | No. of cycles | |||
| First round | K13_PCR_F | CGGAGTGACCAAATCTGGGA | 98°C | 10 seconds | 1 |
| K13_PCR_R | GGGAATCTGGTGGTAACAGC | 98°C | 5 seconds | 35 | |
| 63°C | 10 seconds | ||||
| 72°C | 45 seconds | ||||
| 72°C | 2 minutes | 1 | |||
| 20°C | ∞ | 1 | |||
| Second round (nested 1) | K13_N1_F | GCCAAGCTGCCATTCATTTG | 98°C | 10 seconds | 1 |
| K13_N1_R | GCCTTGTTGAAAGAAGCAGA | 98°C | 5 seconds | 35 | |
| 62°C | 10 seconds | ||||
| 72°C | 25 seconds | ||||
| 72°C | 2 minutes | 1 | |||
| 20°C | ∞ | 1 | |||
| Second round (semi-nested 2) | K13_N2_F | CGCCAGCATTGTTGACTAAT | 98°C | 10 seconds | 1 |
| K13_PCR_R | GGGAATCTGGTGGTAACAGC | 98°C | 5 seconds | 35 | |
| 65°C | 10 seconds | ||||
| 72°C | 35 seconds | ||||
| 72°C | 2 minutes | 1 | |||
| 20°C | ∞ | 1 | |||
Purified PCR products were sequenced according to the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) protocol. Raw nucleotide sequences were assembled using the Lasergene package version 15.0 (DNASTAR Inc., Madison, WI), and the contiguous sequence (1950 bp) was analyzed for the presence of substitutions known to confer resistance to artemisinin. Two amino acid substitutions (R561H and K189T) were found within the PfK13 gene. Of these, R561H substitution is known to be associated with artemisinin resistance in Southeast Asia.8 K189T substitution within the non-propeller region of the PfK13 has previously been reported more commonly in African P. falciparum isolates than in Asian strains, and its phenotypic association with artemisinin resistance has not been validated.9 The timeline of important clinical events is shown in Figure 1.
Figure 1.
The timeline of important clinical events.
DISCUSSION AND CONCLUSION
This report highlights probable recrudescence, in the presence of PfK13 mutations, in a Sri Lankan appropriately treated with ACT for imported falciparum malaria. Artemisinin resistance, defined as delayed parasite clearance after therapy associated with P. falciparum Kelch 13 propeller domain mutations, is widespread in the Greater Mekong Subregion.2,3 PfK13 mutations previously associated with delayed clearance in southeast Asia have been reported at a prevalence greater than 5% in studies from Guyana, Papua New Guinea, and Rwanda.3
In the case reported here, complete clearance of parasites by D3 was seen after treatment with both artemether–lumefantrine and dihydroartemisinin–piperaquine. The patient may have had a submicroscopic infection that may have been undetected on D3 and on follow-up after the primary infection. Lumefantrine blood levels were not checked on D7 because of unavailability of such facilities in this country. As he had been given a weight-appropriate dose of artemether–lumefantrine under direct observation, after a fatty meal, and there was no report of vomiting, reduced exposure to lumefantrine causing treatment failure was unlikely.10
This patient had been to DRC, one of the 10 African nations with highest malaria burden as recognized by the WHO.3 R561H, a marker of artemisinin resistance has been reported in DRC in the past,11,12 but in a more recent study, this marker was not observed.12 The mutations in PfK13 that are known to cause delayed parasite clearance are rarely reported from Africa.13 However, an emergence and clonal expansion of indigenous parasites harboring the R561H mutation was discovered in Rwanda.13 Therefore, in considering the current epidemiology, it is likely that he would have been infected with malaria during his short visit to Rwanda, although it is not possible to confirm this without additional genomic analysis.
Sri Lanka eliminated malaria in 2016, but remains vulnerable as 378 imported cases and one introduced case (probably due to local transmission) of malaria have been reported between 2013 and 2019.14 The presence of primary vector Anopheles culicifacies and the recent spread of the urban vector Anopheles stephensi increase the risk of onward transmission.15 With the prevailing dengue epidemic (105,049 cases reported in 2019),16 malaria is often not suspected in febrile patients. A full blood count is usually performed in febrile patients as per the dengue management guidelines.17 The high prevalence of thrombocytopenia with both dengue and malaria challenges diagnosis (Karunaratna et al., unpublished data).
The Anti-Malaria Campaign (AMC) of Sri Lanka conducts programs to prevent the re-establishment of malaria in the country. These include parasitological and entomological surveillance, regular in-service training programs for laboratory staff of public and private sectors and clinician and public awareness programs. Travelers to endemic countries are advised regarding the prevention of mosquito bites, and chemoprophylaxis is issued free of charge. Travelers to African destinations are mandated to obtain the yellow fever vaccine from the Sri Lanka Ports Health Authority, who also directs them to the AMC to obtain chemoprophylaxis. Moreover, travelers are provided chemoprophylaxis from the health office situated at the Bandaranaike International Airport before departure. Despite these, many individuals do not obtain them.
Messages that Sri Lanka is “malaria free” and to seek immediate medical attention on developing fever on return are displayed at the airport. Once diagnosed, mandatory admission to hospital for treatment ensures the rational use of antimalarials, thus slowing the emergence of resistance. Antimalarials, stocked and distributed under the sole discretion of the AMC, are adequately available at its headquarters and regional offices. The well-developed road network ensures availability of antimalarials at any hospital island-wide within 2 hours.
The response to antimalarials among imported malaria patients in Sri Lanka was first studied in 2015–2016. Three patients of 28 infected with P. falciparum exhibited late treatment failure. Appropriate response was seen when the treatment given for the first infection was repeated.18 The first report of R561H allele in a Rwandan came from Zhejiang Province in China, where molecular surveillance was performed in patients with imported malaria.19 This is the first time molecular markers have been used in a patient with imported malaria diagnosed in Sri Lanka. Monitoring of molecular markers and drug efficacy of currently used antimalarials will continue to be carried out by the AMC on any patient with suspected treatment failure. This will enable early detection and prediction of drug-resistant malaria to prevent dire sequelae.
ACKNOWLEDGMENTS
We thank Harshini Vitharana, Medical Officer, Anti Malaria Campaign, for the support extended in gathering the history of this patient. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1.World Health Orgnization , 2015. Guidelines for the Treatment of Malaria , 3rd edition. Geneva, Switzerland: WHO. Available at: https://www.who.int/malaria/publications/atoz/9789241549127/en/. Accessed October 18, 2020. [Google Scholar]
- 2.World Health Organization , 2020. Q&A on Artemisinin Resistance. Geneva, Switzerland: WHO. Available at: https://www.who.int/malaria/media/artemisinin_resistance_qa/en/. Accessed October 19, 2020. [Google Scholar]
- 3.World Health Organization , 2019. World Malaria Report 2019. Geneva, Switzerland: WHO. Available at: https://www.who.int/publications/i/item/9789241565721. Accessed October 2, 2020. [Google Scholar]
- 4.World Health Organization , 2020. Antimalarial Drug Efficacy and Drug Resistance. Geneva, Switzerland: WHO. Available at: https://www.who.int/malaria/areas/treatment/drug_efficacy/en/. Accessed October 19, 2020. [Google Scholar]
- 5.Anti Malaria Campaign, Ministry of Health, Sri Lanka , 2014. Guidelines on Malaria Chemotherapy and Management of Patients with Malaria. Available at: http://amc.health.gov.lk/Circulars/Treatment-guidelines_Malaria.pdf. Accessed October 2, 2020. [Google Scholar]
- 6.Anti Malaria Campaign, Ministry of Health, Sri Lanka , 2016. Scope of Work to Be Performed when a Malaria Patient is Reported. Available at: http://www.malariacampaign.gov.lk/index.php/en/resources/guidelines. Accessed October 19, 2020. [Google Scholar]
- 7.Ariey F, et al. 2016. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fairhurst RM, Dondorp AM, 2016. Artemisinin-resistant Plasmodium falciparum malaria. Microbiol Spectr 4. 10.1128/microbiolspec.EI10-0013-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaw MT, Emran NA, Lin Z, 2018. Updates on k13 mutant alleles for artemisinin resistance in Plasmodium falciparum. J Microbiol Immunol Infect 51: 159–165. [DOI] [PubMed] [Google Scholar]
- 10.Koru O, Yazici E, Rasmussen C, Ringwald P, Artuk C, Bedir O, 2019. False resistance after artemether–lumefantrine treatment in a falciparum malaria patient in Turkey: a case report. IDCases 1: e00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ménard D, et al. 2016. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med 374: 2453–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yobi DM, Kayiba NK, Mvumbi DM, Boreux R, Bontems S, Kabututu PZ, De Mol P, Speybroeck N, Mvumbi GL, Hayette MP, 2020. The lack of K13-propeller mutations associated with artemisinin resistance in Plasmodium falciparum in Democratic Republic of Congo (DRC). PLoS One 15: e0237791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uwimana A, et al. 2020. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med 26: 1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karunasena VM, et al. 2019. The first introduced malaria case reported from Sri Lanka after elimination: implications for preventing the re-introduction of malaria in recently eliminated countries. Malar J 18: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gayan Dharmasiri AG, Perera AY, Harishchandra J, Herath H, Aravindan K, Jayasooriya HTR, Ranawaka GR, Hewavitharane M, 2017. First record of Anopheles stephensi in Sri Lanka: a potential challenge for prevention of malaria reintroduction. Malar J 16: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epidemiology Unit, Ministry of Health SL , 2020. Dengue Update. Available at: http://www.epid.gov.lk/web/index.php?option=com_content&view=article&id=171%3Adengue-update&catid=51%3Amessage-for-public&Itemid=487&lang=en. Accessed September 24, 2020. [Google Scholar]
- 17.Ministry of Health Sri Lanka , 2012. National Guidelines- Guidelines on Management of Dengue Fever and Dengue Haemorrhagic Fever in Adults. Available at: http://epid.gov.lk/web/images/pdf/Publication/guidelines_for_the_management_of_df_and_dhf_in_adults.pdf. Accessed October 2, 2020. [Google Scholar]
- 18.Dharmawardena P, Rodrigo C, Mendis K, Gunasekera WMKT De AW, Premaratne R, Ringwald P, Fernando D, 2017. Response of imported malaria patients to antimalarial medicines in Sri Lanka following malaria elimination. PLoS One 12: e0188613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Ruan W, Zhou S, Huang F, Lu Q, Feng X, Yan H, 2020. Molecular surveillance of Pfcrt and k13 propeller polymorphisms of imported Plasmodium falciparum cases to Zhejiang province, China between 2016 and 2018. Malar J 19: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]