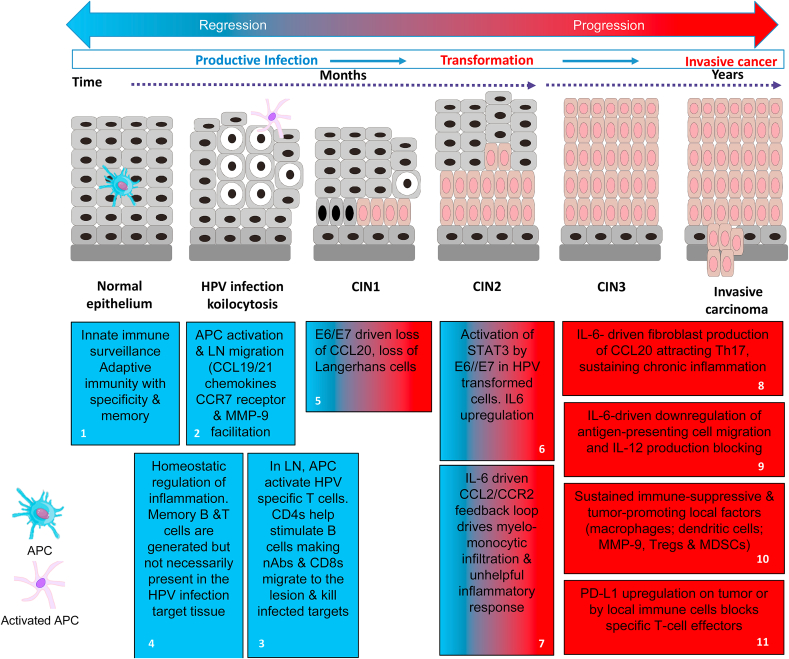
Fig. 1.
Immune control or immune deviation in HPV infection and neoplasiaImmune Control: (blue boxes). Blue box 1: APC detect tissue damage or the presence of HPV through sensor systems (e.g. TLRs) and act as the pivotal messengers to elicit adaptive immunity. Blue box 2: Induction of adaptive immunity requires migration of activated APCs to the local lymph nodes (LN) [1]. This occurs in response to gradients of the chemokines CCL19/CCL21 through CCR7 receptors on the activated APCs [2], plus production of MMP-9 facilitating passage through the extracellular matrix [3]. Blue box 3: APCs process and present viral antigens to activate specific T cells requiring CD80/CD86 and CD28 co-stimulation plus secretion of enabling cytokines like IL-12; without such co-stimulation immune tolerance and T-cell anergy can result [4,5]. Optimally activated T cells multiply and differentiate across a spectrum of associated cytokine production and effector properties to engage the viral threat. For example, helper CD4 T cells facilitate specific B cell stimulation leading to secretion of highly virus specific neutralising antibodies while also supporting the differentiation of CD8 cytotoxic T cells (CTL) able to kill virus infected cells directly or through the secretion of cytokines like IFN and tumour necrosis factor (TNF). Other types of T cell such as regulatory cells (Tregs) and/or the balance of cytokines produced can act to limit local responses to prevent tissue damage [6]. Blue box 4: This is a dynamic process, as when the viral threat is reduced, inhibitory signals (immune checkpoints) between T cells and APCs mediated by CTLA-4/CD28 and PD-1/PD-L1-type interactions act to induce a contraction of the specific effector T cell populations [7,8]. Earlier in the infection, subsets of memory B and T cells are produced so that the immune system can respond faster and more effectively upon the specific virus reinfection [9,10].
Immune Deviation: (blue & red or red boxes) reflect decreasing potential for immune control and increasing immune suppression respectively). Blue/red box 5: Some cases of HPV infection, E6/E7 expression can lead to loss of local APC (Langerhans cells) through down-regulation of the chemokine CCL20 [[33], [34], [35]]. This event inhibits innate immune detection of the viral infection and promotes viral persistence. Blue/red boxes 6 &7: E6/E7 upregulation of STAT-3 in HPV associated cells induces IL-6 can drive a myelo-monocytic cell lesion infiltration through the CCL2/CCR2 axis which is self-reinforcing [[42], [43], [44]]. This type of inflammation is able to provide a significantly immunosuppressive environment further facilitating viral persistence. With time, viral integration can occur with transformation of the cells breaking the link with the epithelial differentiation required for completion of the virus lifecycle. Viral inhibition of DNA repair allows for variants with additional means for immune escape. Red boxes 8 & 9: The emerging tumour microenvironment evolves through multiple IL-6 mediated influences including tumour fibroblast production of CCL20 attracting Th17 cells and the downregulation of antigen-presenting cell migration and IL-12 production blocking immune activation and chronic inflammation [46]. Red boxes 10 & 11: The TME accumulates high levels of immune-suppressive and tumour-promoting local factors including macrophages and dysfunctional dendritic cells: local MMP-9 production, Tregs and myeloid-derived suppressor cells (MDSCs) [31,57]. This milieu of immune players and factors can up-regulate PD-L1 by tumour or the associated immune cells thereby blocking anti-tumour specific T-cell effectors [47].