Abstract
The cornea is a highly specialized transparent tissue located at the anterior most surface of the eye. It consists of three main layers, the outer stratified squamous epithelium, the inner endothelium, and the intermediate stroma. Formation of these layers during development involves a complex interaction between ectodermal-derived structures, such as the overlying head ectoderm, and the periocular mesenchyme (POM)1, the latter of which is comprised of neural crest cells (NCC) and mesoderm derived progenitor cells. Regulation of corneal epithelial development, including both epithelial cell fate and stratification, has been shown to depend on numerous bi-directional mesenchymal-epithelial signaling pathways. In this review we pay particular attention to the genes and signaling pathways that involve the POM.
Keywords: Cornea, epithelium, development, stem cell, neural crest cells, periocular mesenchyme, Wnt/β-catenin, knockout mouse
1. Overview of Corneal Development
The vertebrate cornea is a highly specialized transparent tissue that covers the anterior surface of the eye and is comprised of three main tissue layers, the outer stratified squamous epithelium, the intermediate stroma, and the inner endothelium. During development the surface ectoderm in the head region thickens and gives rise to bilateral lens placodes that invaginate to form lens pits (Beebe and Coats, 2000) (Figure 1). Once the lens pits separate from the overlying ectoderm, the remaining ectoderm will give rise to the presumptive corneal epithelium. Initially, the corneal epithelium is a two layered structure, which at the end of the embryonic period, or post-natally for some species such as mice, will later expand into a 6 to 8 cell layered stratified squamous non-keratinized epithelium (Lwigale, 2015; Zieske, 2004). The corneal stroma and endothelial layers are derived from a different source, the periocular mesenchyme (POM), which is comprised of cranial neural crest cells (NCC) (Johnston et al., 1979). NCC originate from the neural tube during development and those that migrate into the ocular region form the POM. In some species, such as birds, humans and reptiles a number of waves of POM will populate the developing anterior segment (Cvekl and Tamm, 2004; Williams and Bohnsack, 2015). Following separation of the lens from the overlying ectoderm, the primitive corneal epithelium secretes an acellular primary stroma, consisting of loosely arranged collagen fibrils (Hay, 1980; Hendrix et al., 1982), which is then followed by two waves of POM migration. The first wave will form the endothelial cell layer, and the second wave invades the primary stroma to form the corneal keratocyte population (Hay, 1980). The formation of the corneal endothelium from the POM in the first wave occurs via a mesenchyme-to-epithelial transition (MET), resulting in a monolayer of cells between the lens epithelium and the overlying corneal epithelium. In the mouse, only a single influx of POM cells occurs at embryonic day 11.5 (E11.5) in the region between the newly separated lens and exterior surface ectoderm (Gage et al., 2008). As embryonic development proceeds, these cells multiply, flatten, and produce loose ECM, differentiating into the mature keratocytes that begin to synthesize and secrete mature extracellular matrix components consisting of collagens types I, V, VI (Fini, 1999; Linsenmayer et al., 1983), as well as keratan sulfate (Funderburgh et al., 2003). These cells contribute to both the corneal stromal and endothelial cells (Cintron et al., 1983), the latter of which are derived from the posterior layer of the POM via MET by E15.5 (Figure 1).
Figure 1.
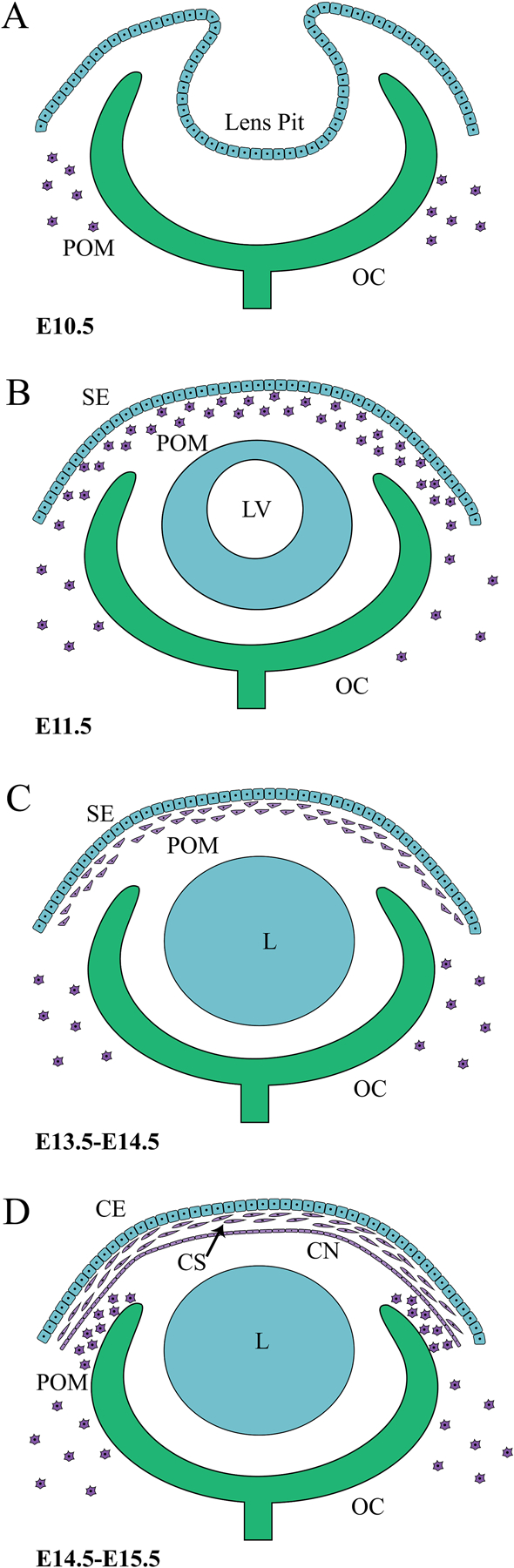
Murine embryonic corneal development and POM migration. (A) The lens pit and optic cup (OC) are formed when the lens placode and optic vesicle invaginate at E10.5. (B) POM cells, derived from the cranial NCCs and mesoderm, migrate at E11.5 into the space between the newly formed surface ectoderm (SE) and lens vesicle (LV). (C) Between E13.5–14.5, while lens fibres close off the lumen of the lens vesicle, the migrated POM cells flatten and condense. (D) By E14.5–15.5, the three layers of the cornea are discernible, with the posterior POM having given rise to the flattened endothelial layer (CN), and outer surface ectoderm developing into the corneal epithelium (CE). The intermediate POM constitutes the corneal stroma (CS) and gives rise to ECM producing keratocytes. Post-E15.5, an additional migration of POM cells along the anterior edge of the optic cup forms the iris stroma and ciliary body stroma. (POM: periocular mesenchyme, OC: optic cup, LV: lens vesicle, SE: surface ectoderm, L: lens, CE: corneal epithelium, CS: corneal stroma, CN: corneal endothelium).
Final stratification of the corneal epithelium in the mouse occurs upon eyelid opening at twelve to fourteen days after birth, and this is in contrast to humans, for which stratification is complete at birth (Lwigale, 2015; Rodrigues et al., 1987; Zhang et al., 2015b). In mice, once the eyelids open, the epithelium rapidly proliferates to become 6–8 cell layers thick as observed in the mature, adult cornea (Zhang et al., 2015b). In contrast, the chick corneal epithelium gradually increases in cell layers, beginning with a 2-cell layered structure that proliferates to 6–8 cell layered epithelium by the time of hatching (Ikeda et al., 1975). The adult corneal epithelium is continuously renewed and maintained by the slow-dividing basal cells, which are themselves derived from a stem cell population in the limbal epithelium. These stem cells give rise to transient amplifying cells (TACs), which proliferate and differentiate, producing terminally differentiated cells (Yazdanpanah et al., 2017). These cells also migrate centripetally from the limbus to the central corneal region, moving apically from basal to suprabasal layers and then to the outer stratified layers. More recent evidence has shown that stem cells are not entirely confined to the limbal region, with some dispersed in the basal layer of the corneal epithelium (Majo et al., 2008).
Corneal development, and in particular, regulation of corneal epithelial cell fate and stratification, is hypothesized to be contingent upon bi-directional mesenchymal-epithelial signaling involving the developing POM. In this review we will pay particular attention to the genes and signaling pathways involving the POM and how this impacts corneal epithelial development.
2. Contribution of the POM to the Developing Cornea
The POM contributes to many structures of the developing eye, including the corneal stroma (Creuzet et al., 2005) and endothelium, as previously outlined. Early research had suggested that the POM arose solely from mesoderm, however fate mapping studies, mainly in birds revealed that NCCs also contribute to the POM. In particular, it has been shown that the NCC aspect of the POM gives rise to the corneal endothelium and stroma, most of the sclera, the trabecular meshwork and the ciliary muscles (Cvekl and Tamm, 2004; Williams and Bohnsack, 2015). Some developmental differences between birds and mammals do however exist. These differences were revealed through detailed studies carried out using binary transgenic systems in mice to permanently label progeny of NCC and mesoderm cells that make up the POM in the eye. Specifically, a Wnt-1cre/R26R labelling system was employed to trace the fates of the NCC in the anterior eye and this was compared with an αGSU-Cre/R26R labelling system that specifically marks mesoderm (Gage, 2005). In this way the NCC and mesoderm portions of the POM contributing to the developing anterior eye were delineated. These experiments revealed that unlike birds, in which the developing stromal cells and endothelial cells are solely derived from NCC, in mice the corneal stromal and endothelial cells appeared to be derived from a mixture of NCC and mesoderm. The authors propose that the difference observed in mammals, with two lineages contributing to the cornea may provide an evolutionary advantage, since the appearance of mesoderm-derived cells in the developing cornea allowed for the formation of dendritic cells and macrophages that have immune surveillance properties.
Numerous transcription factors that are known to be important for development of the cornea are expressed in the POM and some in both the NCC and mesoderm lineages. For example, Pitx2 and Foxc1are expressed in both the NCC and mesoderm POM populations (Gage et al., 2005). Interestingly, mutations in each of these regulators results in similar human disorders, such as Axenfeld-Rieger syndrome that include defects in the cornea (Tumer and Bach-Holm, 2009). Pitx2-deficient mice also exhibit corneal defects such as abnormal corneal stromal and endothelial differentiation (Evans and Gage, 2005). However, the expression of transcription factors in each of the POM lineages may be regulated differently. For example, it was shown that activation of Pitx2 expression in the NCC-derived POM occurs upon their migration into the developing anterior segment as the cells come in proximity to the overlying lens epithelium, the latter of which is presumed to be the signaling center for corneal stromal and endothelial specification (Gage et al., 2005). In contrast the activation in Pitx2 expression in the mesoderm-derived POM population appeared before the cells interacted with the developing ocular ectoderm and was thus independent from the patterning induced in the NCC-derived POM. This differential regulation of transcriptional regulators in the POM lineages may be important to consider to further understand the role of the POM in corneal development and disease. Further utilization of the Wnt-1cre and αGsu-Cre transgenes to generate neural-crest and mesoderm specific knockouts will facilitate this.
3. Bi-directional Signalling in Corneal Epithelial Development
a. Wnt/β-catenin
i. Cell Fate Determination
The POM contributing to the developing corneal stroma and limbal cell stromal niche has been shown to play important roles in specifying both the phenotype and stratification of the corneal epithelium. A number of signaling pathways have been identified as having critical roles in the bi-directional POM-epithelial signaling during corneal development, including the TGF-β and retinoic acid (RA) pathways, and most significantly, if not essentially, the canonical Wnt/β-catenin pathway (Ma and Lwigale, 2019). Canonical Wnt/β-catenin signaling is initiated by the binding of Wnt ligands to a heterodimeric cell surface receptor; these receptors consist of a Frizzled (FZD) subunit and a LRP5/6 subunit (Logan and Nusse, 2004). This binding initiates an intracellular pathway that stabilizes β-catenin and induces its translocation to the nucleus where it regulates expression of various target genes. One key regulatory relationship that has been identified to play an essential role in determining corneal epithelial cell fate is between the canonical Wnt/β-catenin signaling pathway and Dickkopf-2 (Dkk2). Dkk2 is an antagonist of canonical Wnt signaling and is expressed downstream of transcription factor Pitx2 (Gage et al., 2008; He et al., 2004; Mao and Niehrs, 2003). Dkk2 is observed in the migrating POM of mice at E11.5 (Monaghan et al., 1999; Mukhopadhyay et al., 2006), and expression is maintained throughout gestation and post-natally (Ang et al., 2004). At E14.5, when all three corneal layers are distinct, RNA in-situ hybridization revealed that Dkk2 is expressed throughout the mesenchyme with high expression in both central and peripheral regions (Gage et al., 2014). In mouse models for which Pitx2 or Dkk2 were conditionally knocked-down in the POM, the corneal epithelium adopts a conjunctival fate by E16.5 (Gage et al., 2014; Gage et al., 2008). For example, it was observed that the corneal epithelial specific marker K12 was absent in all regions of the epithelium, while the conjunctival marker K4 was present throughout the central cornea (Gage et al., 2014; Gage et al., 2008). Additionally, conjunctival features such as mucus secreting goblet cells and ectopic stromal blood vessels were observed in the corneal region (Gage et al., 2014; Gage et al., 2008). Interestingly, at P10, transcription factor Pax6, which has been studied at length in conjunction with canonical Wnt signaling, and also plays a role in controlling epithelial cell fate, was found to be absent in the developing corneal epithelium of the Dkk2 KO model, unlike wild-type (WT) mice in which expression was maintained in basal epithelial cells (Mukhopadhyay et al., 2006). This suggests that activation of Wnt signaling in the absence of Dkk2, leads to Pax6 suppression, and subsequent loss of corneal specific gene expression (Mukhopadhyay et al., 2006). Other studies have determined that Pax6 directly binds to the promoter of K12, highlighting its necessity in specifying a corneal epithelial phenotype (Kitazawa et al., 2017; Shiraishi et al., 1998). It has also been established that in cases of severe ocular disease, deletion of Pax6 results in loss of K12, and also in increased K10, the latter of which is a marker for epidermal/conjunctival tissue (Li et al., 2008).
Given the antagonistic role of Dkk2, it is suspected that its knock-out allows for activation of Wnt signaling in the central cornea surface ectoderm and POM. Indeed, based on RNA in-situ hybridization, Axin2, a downstream target of Wnt signaling was found to be upregulated in the POM and epithelium by E15.5 in the Dkk2 KO model (Gage et al., 2008). Similarly, in the Pitx2 KO model, as early as E13.5, Axin2 expression was observed throughout the surface ectoderm, in contrast to the WT where it remained confined to the conjunctiva (Gage et al., 2014; Gage et al., 2008). Together, these findings suggest that repression of the Wnt signaling through activation of Dkk2 in the POM is essential for proper specification of the corneal epithelial cell fate/phenotype (Figure 2).
Figure 2.
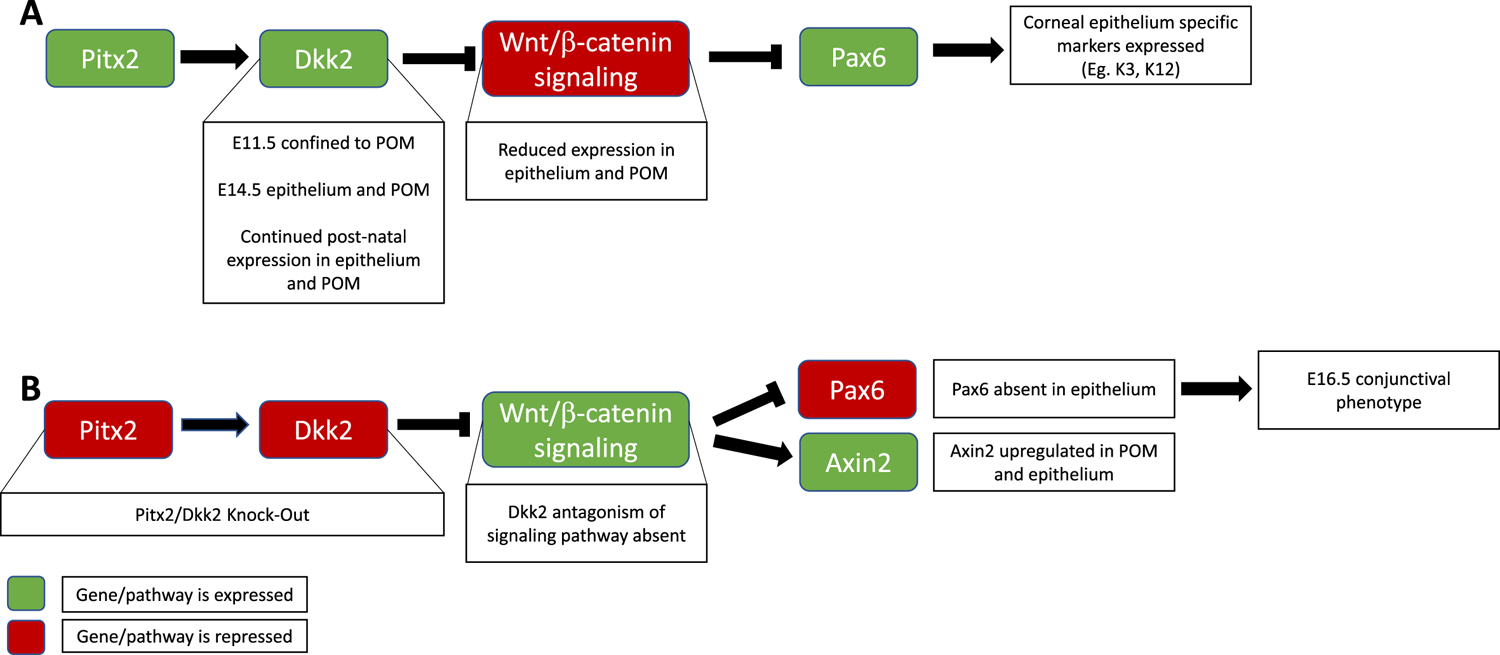
The role of the canonical Wnt/β Catenin pathway in bi-directional signaling determining corneal epithelial cell fate. (A) In the WT mouse, transcription factor Pitx2 is necessary for the expression of Dkk2, which is confined to the mesenchyme at E11.5, but present in both the epithelium and POM at E14.5, and continues to be expressed in these tissues post natally. Dkk2 from these sources antagonizes Wnt/β catenin signaling in the epithelium and POM. Pax6 expression is observed in the epithelium and leads to the expression of corneal specific markers, including K3 and K12. (B) When Dkk2, or the upstream Pitx2, are genetically knocked-down, Wnt/β catenin signaling persists without Dkk2 antagonism. Upregulation of markers of Wnt signaling such as Axin2 are observed in the POM and epithelium, and Pax6 is suppressed in the epithelium. Due to an absence of Pax6, a conjunctival phenotype is observed as early as E16.5.
ii. Cell Stratification
In addition to its role in determining cell phenotype, the canonical Wnt signaling pathway is also implicated in corneal epithelial stratification. In mice, normal epithelial stratification is very limited until eyelid opening at P12–14, being only 1–2 cell layers thick at P0, and 2–3 layers by P10 (Zhang et al., 2015b). Using a knock-in Axin2lacZ model as a reporter for Wnt signaling by X-gal staining, Zhang et al., found that concurrent with epithelial stratification, Wnt/β-catenin signaling in the POM derived stroma gradually decreased from P1–21 (Zhang et al., 2015b). For example, at P1, while there was positive X-gal staining for β-catenin in all three layers of the cornea, only expression in the epithelium remained consistent from P1–21, whereas in the POM-derived stroma, β-catenin was greatly reduced by P7, and absent by P21 (Zhang et al., 2015b). Direct evidence for the role of Wnt signalling in corneal epithelial stratification stems from genetic mouse models in which β-catenin has been specifically targeted. For example, β-catenin loss-of-function (in POM) studies resulted in a thicker corneal epithelium, while gain-of-function led to impaired corneal epithelial stratification (Zhang et al., 2019; Zhang et al., 2015b). In particular, a triple transgenic mouse line in which the β-catenin (Ctnnb1) gene was conditionally knocked out in the POM-derived stroma upon dox administration led to hyperstratification of the corneal epithelium, with mice exhibiting an epithelium consisting of 4–5 layers at P10, relative to 2–3 layers in control mice (Zhang et al., 2015b). Further research using a gain-of-function model with conditional overexpression of a stabilized β-catenin in stromal keratocytes reported the opposite effect, with stratification limited to only 1–2 epithelial cell layers by P21, unlike wild-type mice with a fully stratified epithelium (Zhang et al., 2019).
Bone morphogenic protein-4 (Bmp4) has previously been studied as a downstream target of the Wnt/β-catenin pathway during development. In mice, the corneal stroma is the main source of Bmp4, with expression increasing from P10 to P21 – the time of corneal epithelial stratification (Zhang et al., 2015b). In the β-catenin KO model, Bmp4 was shown to be significantly upregulated in the mutant stroma and epithelium at P10 relative to WT littermates (Zhang et al., 2015b). Mice treated with recombinant Bmp4 every other day from P0 to P8, exhibited early stratification (at P10), with 3–4 epithelial cell layers, relative to 1–2 layers in the PBS treated mice (Zhang et al., 2015b). This highlights the capacity of Bmp4 to induce stratification, and the essential inhibitory role of Wnt signaling prior to normal stratification beginning at P12. Further evidence by Zhang et al., has shown that Bmp4 expression initiates ERK1/2 and Smad1/5 phosphorylation in the basal corneal epithelial cells, resulting in p63 expression (Zhang et al., 2015b). These conclusions are based on immunofluorescence data showing phosphorylation of ERK1/2 and Smad1/5 in response to Bmp4 treatment. Additionally, in β-catenin KO mice, transcription factor p63 is observed to be significantly upregulated in the basal epithelium of the mutant as early as E16.5, and this co-localizes with increased expression of both phosphorylated Smad1/5 and ERK1/2 (Zhang et al., 2015b). Importantly, expression of both Bmp4 and p63 is observed to be downregulated in the mutant overexpressing β-catenin, corresponding with decreased stratification (Zhang et al., 2019). Overall these findings suggest that, prior to eyelid opening, active Wnt/β-catenin signaling is needed in the stroma in order to suppress Bmp4 expression, and thus to prevent epithelial stratification. However, Wnt/β-catenin signaling must be gradually reduced as development approaches P12, so that upon eyelid opening, Bmp4 is upregulated and thereby promotes stratification of the epithelium via ERK1/2 and Smad 1/5 phosphorylation (Figure 3).
Figure 3.
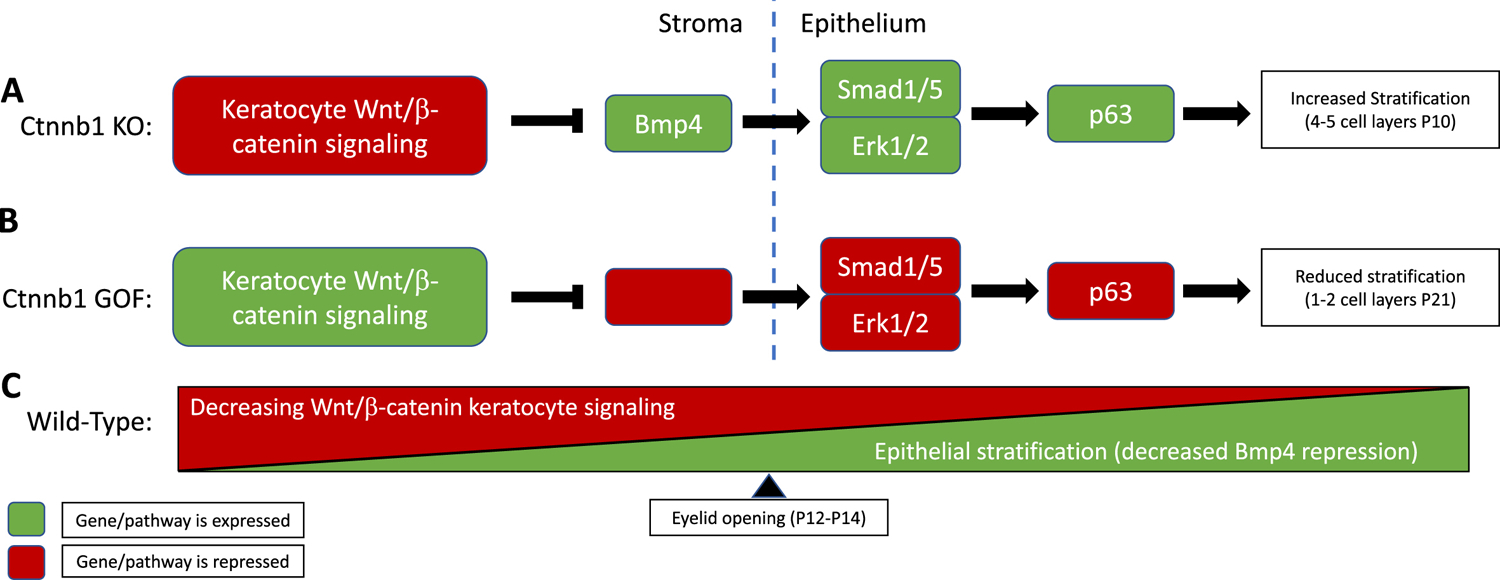
The role of Wnt/β-catenin bi-directional signaling in corneal epithelial stratification. (A) When β-catenin is genetically knocked-down in stromal keratocytes, repression of Bmp4 in the stroma by this pathway is removed. Bmp4 promotes phosphorylation of Smad1/5 and Erk1/2 in the basal corneal epithelial cells, which in turn leads to increased expression of p63, a stem cell marker involved with cell proliferation/stratification. Increased stratification is observed in this model, with 4–5 cell layers by P10. (B) In contrast, in gain-of-function genetic models in which β-catenin is overexpressed by stromal keratocytes, reduced stratification is observed, with only 1–2 cell layers at P21. Bmp4 expression is repressed in the stroma by Wnt signaling, and no longer functions as a paracrine growth factor. The resulting effect is a downregulation of p63 in the epithelium. (C) Epithelial stratification is prevented during early development through Wnt/β -catenin signaling repression of Bmp4. As eyelid opening approaches at P12-P14, Wnt signaling becomes increasingly reduced, allowing for Bmp4 expression in the stroma, resulting in initiation of stratification. Adapted from: (Zhang et al., 2015b).
iii. Limbal Stem Cells
While ligands and receptors for Wnt/β-catenin signaling are expressed throughout the whole corneal epithelium/surface ectoderm, the activity of this pathway appears to be more active the peripheral, limbal regions as compared to the central region (Gage et al., 2008; Liu et al., 2003). The limbal region of the cornea is also of specific interest to development due to the presence of limbal epithelial stem cells in the basal layer and the unique limbal stroma that supports this cell population (Yazdanpanah et al., 2017). Epithelial stem cells, with slow cycling properties and high proliferative capacities, can be found throughout the basal corneal epithelium when stratification begins at approximately 2 weeks of age in mice. A study from Sartaj et al., used an inducible transgenic “pulse-chase” murine model (K5Tta x TRE-H2BGFP) to monitor retention of GFP labeling as a means of identifying the location of stem cells based on their slow cycling properties (Sartaj et al., 2018). They observed that the GFP-positive cell population continually decreased and became more restricted to the basal limbal region, with the 53-day chase period, in the tenth week of age, being the first point at which these cells were almost exclusively limbal (Sartaj et al., 2018). This study also explored the differential genetic profiles of GFP-positive, potential stem cells, and GFP-negative cells (Sartaj et al., 2018) and found that by nine weeks of age, 18.58% of genes were differentially expressed between the two populations. Among these genes are members of the Wnt signaling pathway. For example, Frizzled-7 (Fzd7), a crucial G-coupled receptor in Wnt/β-catenin signaling, is absent from the central cornea, but co-stains with GFP in the basal cells of the limbus (Sartaj et al., 2018). The upregulation of this receptor in limbal stem cells indicates a capacity for increased Wnt signaling relative to the central cornea. Ligands of a mesenchymal or epithelial origin may therefore bind to Fzd7 and initiate the Wnt pathway in limbal stem cells, which in turn may regulate stem cell activity, or perhaps lead to further signaling in the underlying mesenchyme. Additionally supporting this notion, RNA-seq analysis of GFP-positive cells showed an upregulation of the Ctnnb1 and Axin2 genes relative to GFP-negative epithelial cells (Sartaj et al., 2018). Both β-catenin and Axin-2 are upregulated by Wnt signaling, and their presence in limbal stem cells suggests a role in maintaining this cell population.
Another gene found to be preferentially expressed in the limbal region is Sry box 9 (Sox9) (Sartaj et al., 2018). The Sartaj et al. study found that Sox9 co-localized with GFP-expressing basal epithelial cells in the mouse limbus, though expression was also seen in basal cells towards the central cornea (Sartaj et al., 2018). Preferential limbal Sox9 expression has also been observed in limbal epithelial cells isolated from human corneas (Menzel-Severing et al., 2018). In basal limbal epithelial cells, Sox9 is largely confined to a cytoplasmic localization, while in the suprabasal limbal cells and corneal epithelial cells, Sox9 has a predominantly nuclear localization (Menzel-Severing et al., 2018). Deletion of Sox9 in cultured human limbal epithelial cells resulted in increased expression of K12 and K3, markers of differentiated corneal epithelium, and decreased K15 and K14, markers of progenitor cells (Menzel-Severing et al., 2018). It has also been observed that these cells exhibited reduced proliferation, based on BrdU tagging, and also showed increased expression of β-catenin, indicating upregulation of Wnt signaling (Menzel-Severing et al., 2018). The authors proposed that Sox9 and Wnt/β-catenin signaling cooperate in a mutually repressive manner to control proliferation and differentiation of limbal epithelial cells (Menzel-Severing et al., 2018). For example, during limbal stem cell quiescence Sox9 remains in the cytoplasm whereas translocation of Sox9 to the nucleus stimulates limbal stem cells to give rise to proliferating TACs. This balance between Sox9 and Wnt signaling may be controlled by ligands expressed in the mesenchyme, such as the previously identified antagonist Dkk2.
b. FGF, RA, and TGF-β
Although the canonical Wnt/β-catenin signaling pathway plays a central role in the context of corneal epithelial differentiation and proliferation, the interactions between the developing POM and epithelium are complex and involve pathways beyond Wnt signaling. Particularly between birth and eyelid opening, when stratification commences, key proteins including Pax6 and Bmp4 are closely, but not exclusively, regulated by Wnt signaling. For example, fibroblast growth factor receptor (FGFR) signaling plays a role in corneal development (Zhang et al., 2015a). Knock-down of FGFR2 in mice, has been shown to result in absence of Pax6, and the corneal marker K12, as well as reduced epithelial stratification (Zhang et al., 2015a). In the stroma, FGFR2 KO mesenchymal cells are also unable to differentiate into mature keratocytes, shown by an absence of keratocan (Zhang et al., 2015a). This finding indicates a role for FGF signaling in epithelial-mesenchymal interactions. FGFR2 is expressed in basal cells of the corneal epithelium and ligands for FGFR2 are expressed by the POM, including FGF7 and FGF10 (Chikama et al., 2008; Igarashi et al., 1998). Thus, based on the effects of FGFR2 KO, the binding of these mesenchymal ligands to their epithelial receptor may be necessary for both differentiation and proliferation of the epithelium.
Another major regulator in corneal development is retinoic acid (RA). Transcriptome profiling of the mouse POM during corneal development found that RA signaling in the POM and embryonic cornea is either autocrine or derived from surrounding structures (Ma and Lwigale, 2019). In the latter case, RA is synthesized by Raldh1 and Raldh3 in epithelial tissues such as the neuroretina, lens and prospective corneal epithelium, exerting its effects on the POM by binding to the RA receptors (RAR) present in these cells (Molotkov et al., 2006). In the POM, nuclear RA receptors, RARα/β/γ are all expressed between E10.5–14.5; however, following this point, only RARα/γ are expressed in the epithelium and stroma (Cvekl and Wang, 2009). Defects in the RA signaling pathway lead to various ocular pathologies that impact the developing cornea. For instance, knock-down of RARα/β receptors in the POM using a Wnt1-cre driver causes a thickened POM layer between the lens and SE, followed by abnormal stromal organization (Matt et al., 2005). Interestingly, RA has been shown to activate transcription factors Pitx2 and Foxc1 in the POM, resulting in an upregulation of Dkk2 and Pax6 (Ma and Lwigale, 2019). Thus, this pathway may indirectly interact with Wnt/β catenin.
TGF-β signaling is often associated with wound healing, but it also plays roles in development, cell migration and differentiation, among other functions. The TGF-β receptor 2 (TGF-βR2) has been identified as a crucial part of TGF-β signaling in the cornea, with TGF-β ligands binding to TGF-βR2 which in turn phosphorylates TGF-βR1, leading to activation of Smad2/3 signaling (Webber et al., 2016). Transcriptome profiling of mouse POM indicates that TGF-βR2 is highly upregulated at E14.5 and E16.5, suggesting it may regulate POM migration (Ma and Lwigale, 2019). TGF-βR2 mutants are unable to phosphorylate Smad2, expression of Foxc1 and Pitx2 is absent, and these mutants present with irregular stromal collagen synthesis and keratocyte differentiation (Ittner et al., 2005). Interestingly, potential cross-talk between TGF-β signaling and canonical Wnt signaling is suspected, given that TGF-β expression leads to an upregulation of Wnt activating genes, such as Hmga2, which localizes to the corneal epithelium (Ma and Lwigale, 2019).
c. AP-2β
Tfap2b, the gene that encodes Activating Protein-2 beta (AP-2β), is expressed in the POM and has also been shown to have an important role in corneal epithelial stratification and cell fate (Chen et al., 2016; Martino et al., 2016). In mice during early eye development (E10.5), Tfap2b is expressed in a few POM cells as well as the overlying ocular ectoderm, however, from E14.5 onward, it becomes highly expressed in the POM and continues to be expressed in the post-natal corneal stroma and endothelium (Chen et al., 2016; Martino et al., 2016). Germline deletion of Tfap2b results in perinatal death (Moser et al., 1997). Thus, the Wnt1-Cre driver was utilized to create a neural crest specific AP-2β KO model (AP-2β NCC KO) that allowed for investigation of the role of AP-2β in the POM during post-natal stages (Martino et al., 2016). The AP-2β NCC KO mice exhibited a unique phenotype, including an absent corneal endothelium, a hyper-cellular and vascularized corneal stroma, and interestingly, very limited corneal epithelial stratification (Martino et al., 2016). Further investigation of this model has shown that epithelial conjunctivalization has occurred, based on the appearance of the conjunctival marker, K15 across the entire corneal epithelial surface rather than solely the limbal region as is seen in WT mice (West-Mays, preliminary observations). These data suggest that loss of AP-2β in the developing POM-derived corneal stroma disrupts the bi-directional signalling required for normal corneal epithelial development. Interestingly, AP-2β has also been shown to be absent in the corneal stroma of Pitx2-KO mice suggesting that it is downstream of Pitx2 and may also be part of the canonical Wnt/β-catenin signaling pathway (Chen et al., 2016).
Conclusions
Corneal development involves a complex interaction between multiple tissue sources including the head ectoderm and the NCC-derived POM. As we have outlined in this review, the POM is key in providing signals to determine both the cell fate and stratification of the corneal epithelium. Numerous signalling pathways are involved in this process such as Wnt, RA and TGFβ, as are the transcription factors Pitx2, Foxc1, Pax6 and AP-2β. When these pathways are perturbed, aberrant corneal phenotypes are produced such as replacement of the corneal phenotype with a conjunctival appearance and lack of corneal epithelial stratification. The fact that mutations in these different pathways causes similar corneal defects suggests that there is a complex interaction between them. Recent transcriptome analyses of the developing cornea have provided further insight into this crosstalk (Ma and Lwigale, 2019), and such analyses will be useful for identifying novel genes and molecular mechanisms involved in both congenital corneal disease and adult corneal disorders.
Highlights.
Corneal development depends on bi-directional signalling between the overlying head ectoderm and the periocular mesenchyme (POM)
The POM is comprised of neural crest cells (NCC) and mesoderm derived progenitor cells
Regulation of corneal epithelial cell fate and stratification has been shown to depend on numerous bi-directional mesenchymal-epithelial signaling pathways
Key signalling pathways involved in corneal development include Wnt/β-catenin, Retinoic Acid, TGFβ, and FGF
Key transcription factors involved in the signalling pathways controlling corneal development include Pitx2, Foxc1, Pax6 and AP-2β
Acknowledgements
The authors thank Dr. Aftab Taiyab, McMaster University, for his helpful suggestions on this manuscript.
Funding
This work was supported by the National Institute of Health (grant number R01 EY025789) (JWM, TW), and the Bright Focus Foundation grant (JWM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations
NCC – Neural crest cell
POM – Periocular mesenchyme
References
- Ang SJ, Stump RJ, Lovicu FJ, McAvoy JW, 2004. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr Patterns 4, 289–295. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Coats JM, 2000. The lens organizes the anterior segment: specification of neural crest cell differentiation in the avian eye. Dev Biol 220, 424–431. [DOI] [PubMed] [Google Scholar]
- Chen L, Martino V, Dombkowski A, Williams T, West-Mays J, Gage PJ, 2016. AP-2βeta Is a Downstream Effector of PITX2 Required to Specify Endothelium and Establish Angiogenic Privilege During Corneal Development. Invest Ophthalmol Vis Sci 57, 1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikama T, Liu CY, Meij JT, Hayashi Y, Wang IJ, Yang L, Nishida T, Kao WW, 2008. Excess FGF-7 in corneal epithelium causes corneal intraepithelial neoplasia in young mice and epithelium hyperplasia in adult mice. Am J Pathol 172, 638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintron C, Covington H, Kublin CL, 1983. Morphogenesis of rabbit corneal stroma. Invest Ophthalmol Vis Sci 24, 543–556. [PubMed] [Google Scholar]
- Creuzet S, Vincent C, Couly G, 2005. Neural crest derivatives in ocular and periocular structures. Int J Dev Biol 49, 161–171. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Tamm ER, 2004. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays 26, 374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Wang WL, 2009. Retinoic acid signaling in mammalian eye development. Exp Eye Res 89, 280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AL, Gage PJ, 2005. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet 14, 3347–3359. [DOI] [PubMed] [Google Scholar]
- Fini ME, 1999. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res 18, 529–551. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Mann MM, Funderburgh ML, 2003. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem 278, 45629–45637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Kuang C, Zacharias AL, 2014. The homeodomain transcription factor PITX2 is required for specifying correct cell fates and establishing angiogenic privilege in the developing cornea. Dev Dyn 243, 1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Qian M, Wu D, Rosenberg KI, 2008. The canonical Wnt signaling antagonist DKK2 is an essential effector of PITX2 function during normal eye development. Dev Biol 317, 310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T, 2005. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci 46, 4200–4208. [DOI] [PubMed] [Google Scholar]
- Hay ED, 1980. Development of the vertebrate cornea. Int Rev Cytol 63, 263–322. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X, 2004. LDL receptor-related proteins 5 and 6 in Wnt/βeta-catenin signaling: arrows point the way. Development 131, 1663–1677. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Hay ED, von der Mark K, Linsenmayer TF, 1982. Immunohistochemical localization of collagen types I and II in the developing chick cornea and tibia by electron microscopy. Invest Ophthalmol Vis Sci 22, 359–375. [PubMed] [Google Scholar]
- Igarashi M, Finch PW, Aaronson SA, 1998. Characterization of recombinant human fibroblast growth factor (FGF)-10 reveals functional similarities with keratinocyte growth factor (FGF-7). J Biol Chem 273, 13230–13235. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Maisel H, Waggoner D, 1975. An immunofluorescent study of cornea development in the chick. J Embryol Exp Morphol 33, 279–290. [PubMed] [Google Scholar]
- Ittner LM, Wurdak H, Schwerdtfeger K, Kunz T, Ille F, Leveen P, Hjalt TA, Suter U, Karlsson S, Hafezi F, Born W, Sommer L, 2005. Compound developmental eye disorders following inactivation of TGFβeta signaling in neural-crest stem cells. J Biol 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MC, Noden DM, Hazelton RD, Coulombre JL, Coulombre AJ, 1979. Origins of avian ocular and periocular tissues. Exp Eye Res 29, 27–43. [DOI] [PubMed] [Google Scholar]
- Kitazawa K, Hikichi T, Nakamura T, Sotozono C, Kinoshita S, Masui S, 2017. PAX6 regulates human corneal epithelium cell identity. Exp Eye Res 154, 30–38. [DOI] [PubMed] [Google Scholar]
- Li W, Chen YT, Hayashida Y, Blanco G, Kheirkah A, He H, Chen SY, Liu CY, Tseng SC, 2008. Down-regulation of Pax6 is associated with abnormal differentiation of corneal epithelial cells in severe ocular surface diseases. J Pathol 214, 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmayer TF, Fitch JM, Schmid TM, Zak NB, Gibney E, Sanderson RD, Mayne R, 1983. Monoclonal antibodies against chicken type V collagen: production, specificity, and use for immunocytochemical localization in embryonic cornea and other organs. J Cell Biol 96, 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA, 2003. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn 227, 323–334. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R, 2004. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20, 781–810. [DOI] [PubMed] [Google Scholar]
- Lwigale PY, 2015. Corneal Development: Different Cells from a Common Progenitor. Prog Mol Biol Transl Sci 134, 43–59. [DOI] [PubMed] [Google Scholar]
- Ma J, Lwigale P, 2019. Transformation of the Transcriptomic Profile of Mouse Periocular Mesenchyme During Formation of the Embryonic Cornea. Invest Ophthalmol Vis Sci 60, 661–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majo F, Rochat A, Nicolas M, Jaoude GA, Barrandon Y, 2008. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 456, 250–254. [DOI] [PubMed] [Google Scholar]
- Mao B, Niehrs C, 2003. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene 302, 179–183. [DOI] [PubMed] [Google Scholar]
- Martino VB, Sabljic T, Deschamps P, Green RM, Akula M, Peacock E, Ball A, Williams T, West-Mays JA, 2016. Conditional deletion of AP-2βeta in mouse cranial neural crest results in anterior segment dysgenesis and early-onset glaucoma. Dis Model Mech 9, 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt N, Dupe V, Garnier JM, Dennefeld C, Chambon P, Mark M, Ghyselinck NB, 2005. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 132, 4789–4800. [DOI] [PubMed] [Google Scholar]
- Menzel-Severing J, Zenkel M, Polisetti N, Sock E, Wegner M, Kruse FE, Schlotzer-Schrehardt U, 2018. Transcription factor profiling identifies Sox9 as regulator of proliferation and differentiation in corneal epithelial stem/progenitor cells. Sci Rep 8, 10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G, 2006. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development 133, 1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan AP, Kioschis P, Wu W, Zuniga A, Bock D, Poustka A, Delius H, Niehrs C, 1999. Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech Dev 87, 45–56. [DOI] [PubMed] [Google Scholar]
- Moser M, Pscherer A, Roth C, Becker J, Mucher G, Zerres K, Dixkens C, Weis J, Guay-Woodford L, Buettner R, Fassler R, 1997. Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2βeta. Genes Dev 11, 1938–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay M, Gorivodsky M, Shtrom S, Grinberg A, Niehrs C, Morasso MI, Westphal H, 2006. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development 133, 2149–2154. [DOI] [PubMed] [Google Scholar]
- Rodrigues M, Ben-Zvi A, Krachmer J, Schermer A, Sun TT, 1987. Suprabasal expression of a 64-kilodalton keratin (no. 3) in developing human corneal epithelium. Differentiation 34, 60–67. [DOI] [PubMed] [Google Scholar]
- Sartaj R, Zhang C, Wan P, Pasha Z, Guaiquil V, Liu A, Liu J, Luo Y, Fuchs E, Rosenblatt MI, 2018. Author Correction: Characterization of slow cycling corneal limbal epithelial cells identifies putative stem cell markers. Sci Rep 8, 2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi A, Converse RL, Liu CY, Zhou F, Kao CW, Kao WW, 1998. Identification of the cornea-specific keratin 12 promoter by in vivo particle-mediated gene transfer. Invest Ophthalmol Vis Sci 39, 2554–2561. [PubMed] [Google Scholar]
- Tumer Z, Bach-Holm D, 2009. Axenfeld-Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur J Hum Genet 17, 1527–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber HC, Bermudez JY, Sethi A, Clark AF, Mao W, 2016. Crosstalk between TGFβeta and Wnt signaling pathways in the human trabecular meshwork. Exp Eye Res 148, 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AL, Bohnsack BL, 2015. Neural crest derivatives in ocular development: discerning the eye of the storm. Birth Defects Res C Embryo Today 105, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanpanah G, Jabbehdari S, Djalilian AR, 2017. Limbal and corneal epithelial homeostasis. Curr Opin Ophthalmol 28, 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Upadhya D, Lu L, Reneker LW, 2015a. Fibroblast growth factor receptor 2 (FGFR2) is required for corneal epithelial cell proliferation and differentiation during embryonic development. PLoS One 10, e0117089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang YC, Okada Y, Zhang S, Anderson M, Liu CY, Zhang Y, 2019. Aberrant expression of a stabilized beta-catenin mutant in keratocytes inhibits mouse corneal epithelial stratification. Sci Rep 9, 1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yeh LK, Zhang S, Call M, Yuan Y, Yasunaga M, Kao WW, Liu CY, 2015b. Wnt/βeta-catenin signaling modulates corneal epithelium stratification via inhibition of Bmp4 during mouse development. Development 142, 3383–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD, 2004. Corneal development associated with eyelid opening. Int J Dev Biol 48, 903–911. [DOI] [PubMed] [Google Scholar]


