Fig 3. CgPst2 is cleaved at the C-terminus.
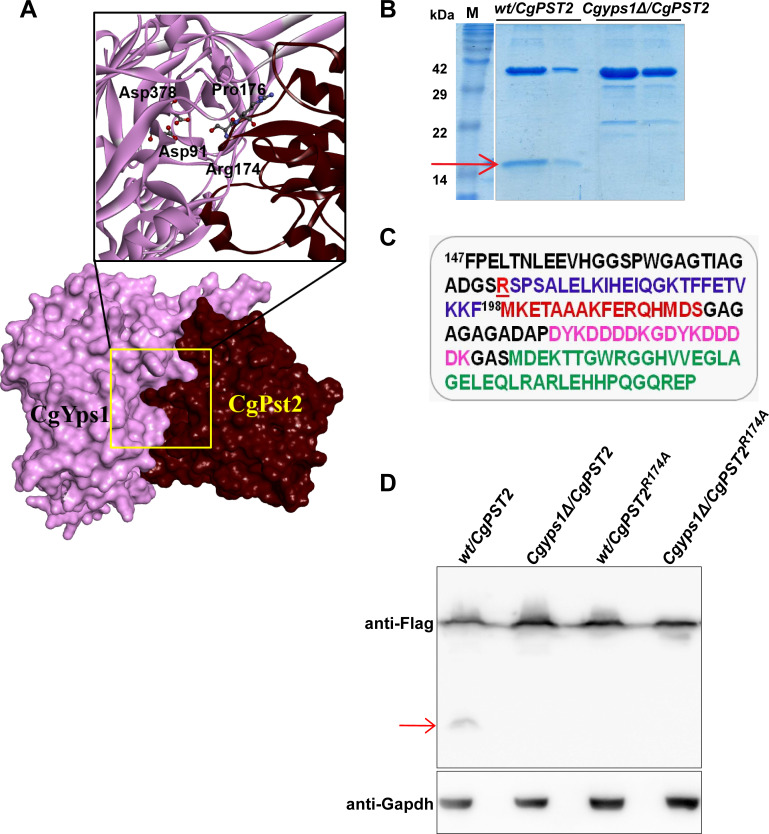
A. Molecular surface and Ribbon models depicting CgYps1 (Length: 601 aa; Predicted MW: 64 kDa) with CgPst2 (Length: 198 aa; Predicted MW: 21 kDa). D91 and D378 in CgYps1 represent catalytically active aspartic acid residues. The 171DGSRSPSA178 region in CgPst2 contains predicted CgYps1-binding residues, Arginine-174 and Proline-176. Hydrogen bond-forming amino acid residues are highlighted in bold letters in the predicted interaction region. The molecular surfaces of receptor (CgYps1) and substrate (CgPst2) are coloured purple and maroon, respectively. B. Coomassie-blue-stained 18% SDS-PAGE gel images indicating expression of the full-length (~ 40 kDa) and the C-terminal cleaved fragment (~ 16 kDa; marked with a red arrow) of CgPst2-SFB, after two-step affinity purification of cell extracts of wt and Cgyps1Δ strains. C. The C-terminus amino acid sequence of CgPst2-SFB protein starting with the phenylalanine (F) residue at 147th position of CgPst2 protein. CgPst2 is 198 amino acid long. The predicted cleavage residue R174 is underlined and indicated in red. The SFB tag (85 aa) consists of S-protein (MKETAAAKFERQHMDS; brown), two copies of the FLAG tag (DYKDDDDK; pink) and streptavidin-binding-peptide (MDEKTTGWRGGHVVEGLAGELEQLRARLEHHPQGQREP; green) sequences. MS analysis of the shorter CgPst2-SFB fragment identified peptides corresponding to SFB and CgPst2 sequences. D. Immunoblot analysis of wt cell extracts expressing either wild-type CgPst2-SFB or alanine-substituted-CgPst2-SFB (CgPST2R174A), using anti-Flag antibody. The red arrow marks the small cleaved fragment of wild-type CgPst2. CgGapdh was used as a loading control.