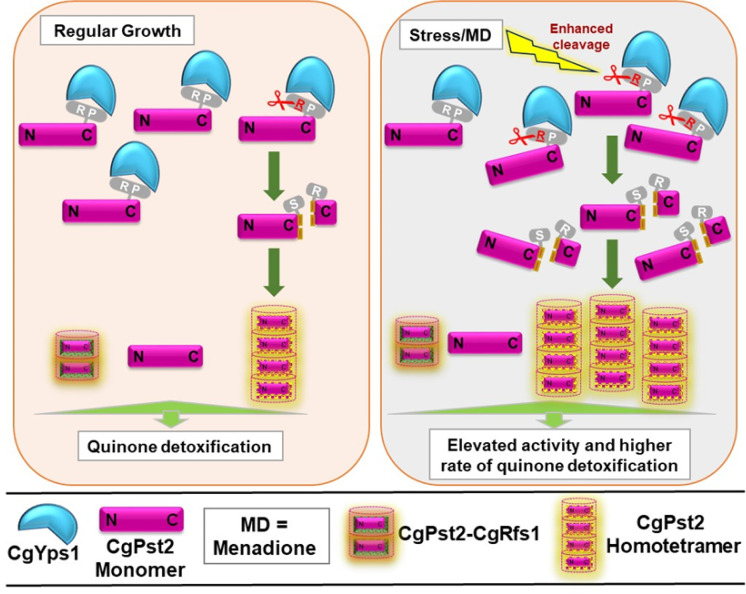
Fig 7. A schematic summarizing key findings of the study.
Arginine-174 (R) and Proline-176 (P) residues of CgPst2 are predicted to interact with CgYps1 at the plasma membrane, and CgYps1 processes R174 residue in the C-terminus of CgPst2. This cleavage, which is elevated upon menadione treatment, leads to removal of the C-terminal domain, resulting in CgPst2 homo-tetramerization, higher activity and efficient quinone detoxification. Of note, the signal, that stimulates CgYps1-mediated cleavage of CgPst2, is not known. CgPst2 also interacts with CgRfs1, however, CgPst2-CgRfs1 association is not dependent on CgYps1, and occurs under both regular and MD treatment conditions. Altogether, CgPst2 functions are regulated at multiple levels, and CgYps1-mediated cleavage of CgPst2 may reflect one of many mechanisms controlling CgPst2 activity.