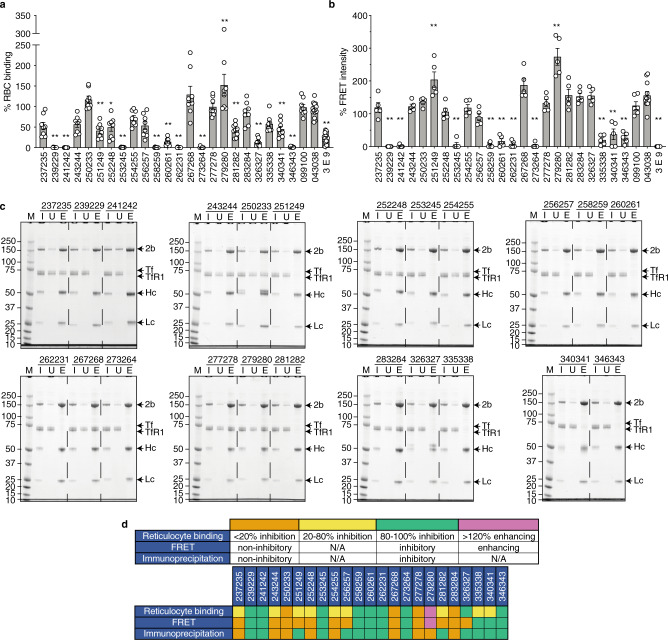
Fig. 2. PvRBP2b human mAbs inhibit PvRBP2b161–1454 binding to TfR1–Tf.
a PvRBP2b161–1454 binding to reticulocytes in the presence of human mAbs was analyzed by flow cytometry. PvRBP2b161–1454 binding in the absence of mAbs was assigned to 100%. 043038 and 099100 were used as antibody isotype controls. 3E9 is an inhibitory PvRBP2b-mouse mAb. n = 8 independent experiments and data are presented as mean ± SEM; circles represent independent experiments. Prism (version 8.4.3) was used to perform one-way ANOVA followed by Dunnett’s multiple comparisons test using 043038 as a control to determine P-values. *P ≤ 0.001, **P ≤ 0.0001. b The ability of PvRBP2b human mAbs to inhibit PvRBP2b161–1454-TfR1–Tf complex formation was analyzed in the FRET-based assay. The FRET intensity in the absence of mAbs was assigned to 100%. n = 5 independent experiments and data are presented as mean ± SEM; circles represent independent experiments. Prism (version 8.4.3) was used to perform one-way ANOVA followed by Dunnett’s multiple comparisons test using 099100 as a control to determine P-values. **P ≤ 0.0001. c IP of PvRBP2b161–1454 using PvRBP2b human antibodies in the presence of TfR1 and Tf were examined by reducing SDS-PAGE. n = 1 independent experiment. 2b, PvRBP2b; Hc, mAb heavy chain; Lc, mAb light chain; I, input; U, unbound; E, eluate; M, molecular weight marker with molecular weights labeled on the left side of the gels in kDa. d Summary table of PvRBP2b human mAb phenotypes in the reticulocyte-binding, FRET, and IP assays. The different phenotypes indicated in orange, yellow, green, and pink for each assay have been shown in the legend. Source data are provided as a Source Data file.