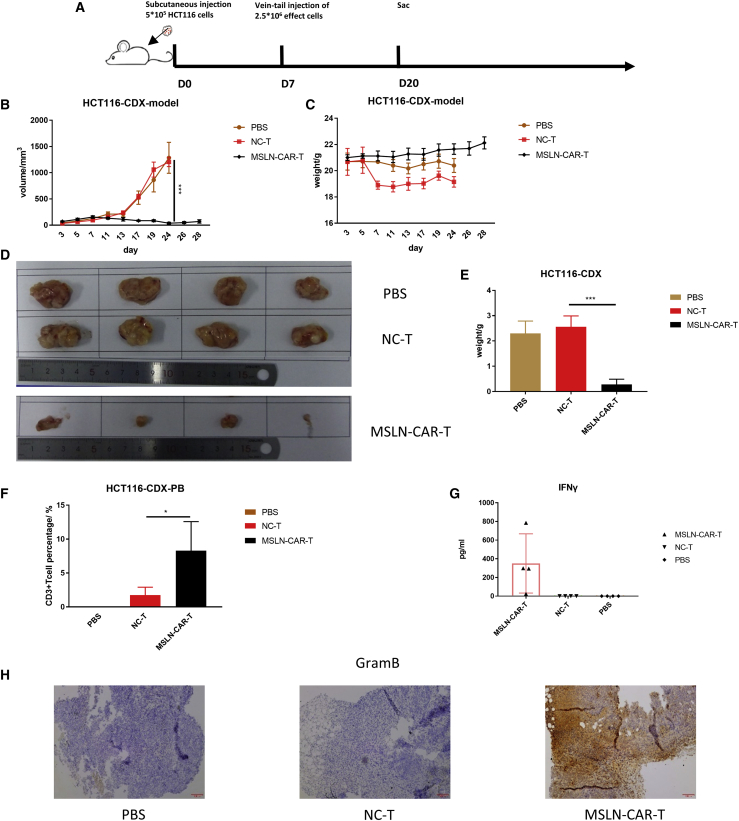
Figure 7.
Anti-tumor efficacy of MSLN-CAR -T cells against colorectal cancer in vivo
(A) Schema of the experimental events and nodes. In this process, model mice were injected with 5 × 105 CRC cells HCT116. 10 days after injection, 12 mice were randomly divided into 3 groups (n = 4): 5 × 106 MSLN-CAR-T cells-treated group, control-T cells-treated group, and PBS of the same volume-treated group. After only one injection, we started observing the mice. (B and C) The tumor size (B) and mice weight (C) variated with MSLN-CAR-T cells, control T cells, or PBS injection among the 28 days. (D) Size of tumor mass differed in MSLN-CAR-T cells, control T cells, or PBS-treated mice. (E) Weight of tumor mass differed in MSLN-CAR-T cells, control T cells, or PBS-treated mice. On day 20, the mice were sacrificed; the CD3+ T cell amount in the PB of HCT116 CDX model is as shown (F). The level of IFN-γ in mouse serum was evaluated by ELISA as shown (G). (H) Immunohistochemistry (IHC) analysis of granzyme B in tumor sections (n = 4, mean ± SEM; p > 0.05, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).