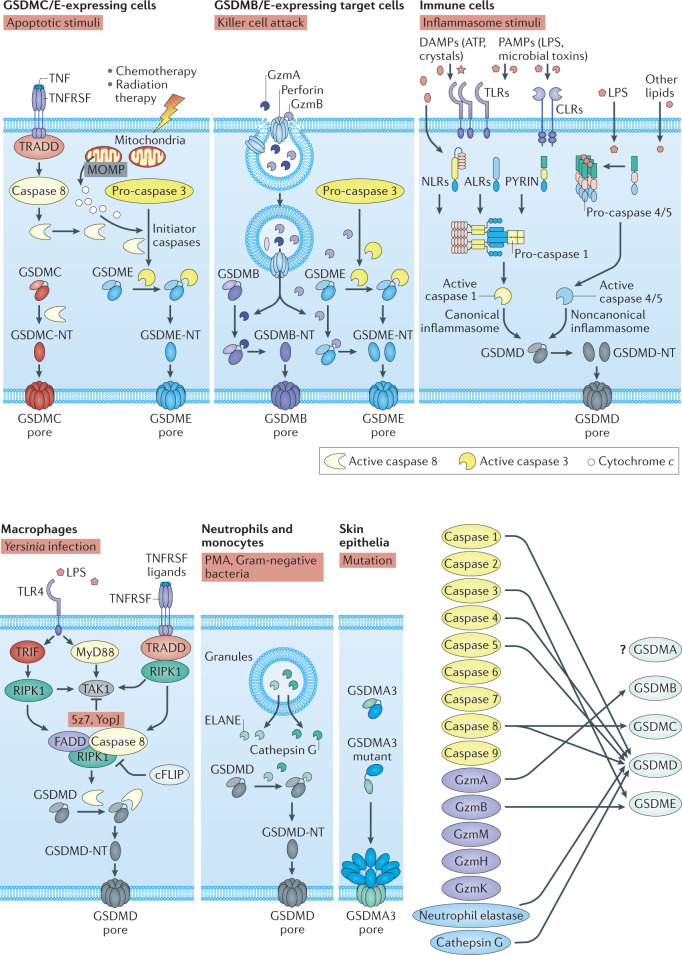
Fig. 3. Molecular mechanisms that activate gasdermins.
In response to apoptotic signalling, gasdermin C (GSDMC) and GSDME are processed by caspase 8 and caspase 3, respectively, converting apoptosis into pyroptosis. Granzymes (Gzms) secreted by killer cells are delivered by perforin into target cells, where GzmA and GzmB can directly cleave and activate GSDMB and GSDME, respectively, to trigger pyroptotic cell death. In immune sentinel cells, both cytosolic canonical inflammasomes that respond to microbial infection or danger signals and the noncanonical inflammasome that responds directly to lipopolysaccharide (LPS) or endogenous oxidized phospholipids activate the inflammatory caspases (caspases 1, 4, 5 and 11), which cleave GSDMD and generate pore-forming N-terminal GSDMD (GSDMD-NT). TAK1 inhibition by the Yersinia effector protein YopJ or the small molecule 5z7 triggers caspase 8-dependent GSDMD cleavage and activation. GSDMD can also be directly processed and activated by neutrophil elastase (ELANE) and cathepsin G. In addition to protease-mediated release of active GSDM-NT, mutations in Gsdma3 lead to abolition of C-terminal GSDM inhibition and trigger GSMDA3 pore-forming activity. Diagram at bottom right indicates the proteases known to cleave and activate each of the gassdermins (yellow, caspases; purple, lymphocyte granzymes; blue, myeloid cell granule proteases). ALR, AIM-2 like receptor; CLR, C type lectin receptor; DAMP, damage-associated molecular pattern; NLR, NOD-like receptor; MOMP, mitochondrial outer membrane permeabilization; PAMP, pathogen-associated molecular pattern; TLR, Toll-like receptor.