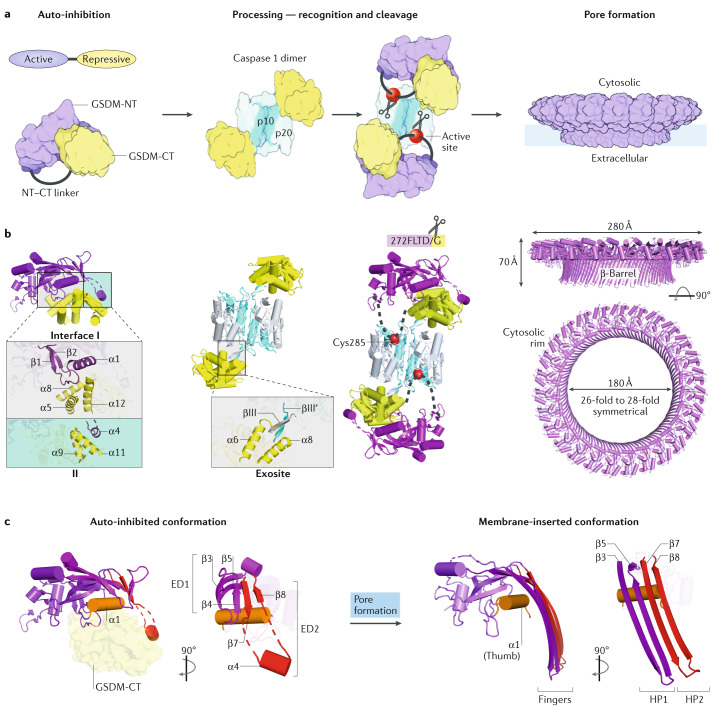
Fig. 4. Mechanisms of gasdermin auto-inhibition, processing and pore formation.
a | Cartoon representation of gasdermin (GSDM) pore formation. Interaction between the N-terminal and C-terminal fragments of a GSDM (GSDM-NT, in purple and GSDM-CT, in yellow) auto-inhibits protein activation. Active caspase 1, a dimer of the p10 (in pale blue)–p20 (in silver) complex, recognizes two copies of GSDM through the GSDM-CT. This exosite recognition mechanism places the long interdomain linker (in grey) between GSDM-NT and GSDM-CT near the active site of caspase 1 (red balls). Active caspase 4 and caspase 11 use a similar mechanism to process GSDMD. Linker cleavage liberates GSDM-NT for pore formation at the membrane. The fully formed GSDM pore features a large cytosolic rim in addition to a transmembrane β-barrel. b | Structural illustration of GSDM at each stage in pore formation depicted in part a. In auto-inhibited GSDM (PDB: 5B5R for GSDMA3), GSDM-NT contacts GSDM-CT at two interfaces. Interface I is formed primarily by charged interactions of the α1 helix and the β1–β2 loop of GSDM-NT with the α5–α8–α12 helix cluster of GSDM-CT. Interface II is formed mainly by hydrophobic interactions between α4 of GSDM-NT and α9 and α11 of GSDM-CT. GSDMD recognition (PDB: 6KN0 and 6VIE) by caspase 1 is mediated by a substrate-binding exosite, comprised of two anti-parallel β-strands — βIII of caspase 1-p20 and βIII′ of caspase 1-p10. The exosite binds to α6 and α8 of GSDMD-CT through primarily hydrophobic interactions. The GSDMD NT–CT linker is positioned near the active site Cys285 of caspase 1 for cleavage. The GSDM pore contains roughly 27 subunits according to the structure of the GSDMA3 pore (PDB: 6CB8). With each subunit contributing four transmembrane β-strands, the membrane-inserted β-barrel contains 108 β-strands. The inner and outer diameters of the pore are approximately 180 Å and 280 Å, respectively. A soluble rim, rich in α-helices and loops, decorates the β-barrel on the cytosolic side. c | GSDM-NT conformations before (left) and after (right) pore formation. The positively charged lipid-binding helix α1 (in orange, like a thumb) is masked by GSDM-CT in the auto-inhibited conformation, and in the membrane-inserted conformation it interacts with lipids with negatively charged head groups found on the inner leaflet of the plasma membrane. Drastic conformational changes of GSDM-NT occur at extension domains 1 and 2 (ED1, in magenta and ED2, in red), which are located at the β3–β4–β5 and β7–α4–β8 regions in auto-inhibited GSDM-NT, respectively. Through pore formation, ED1 and ED2 become the two transmembrane β-hairpins (HP1, in magenta and HP2, in red, like four fingers), respectively.