FIGURE 4.
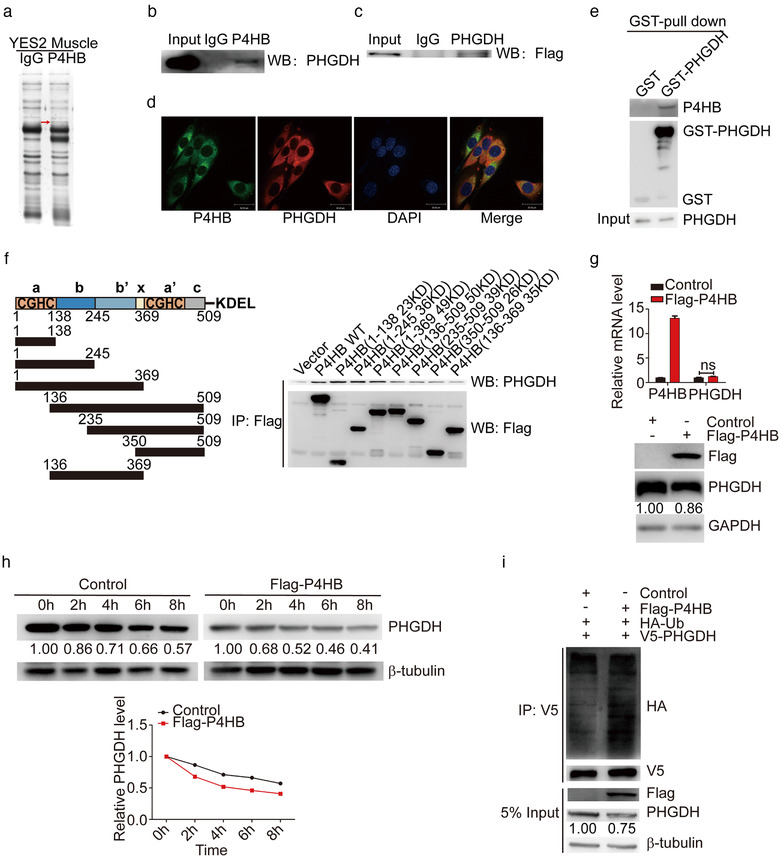
P4HB interacts with PHGDH and destabilizes PHGDH by increasing its ubiquitination level. (a) Identification of P4HB‐interacting proteins. Proteins extracted from GA muscle of YES2‐bearing mice were incubated with Protein A/G Sepharose beads conjugated with anti‐P4HB antibody, subjected to SDS‐PAGE and visualized by silver staining. The band indicated by the red arrow was subjected to mass spectrometry. (b) Endogenous PHGDH detected by the co‐immunoprecipitation assay with anti‐P4HB antibody in C2C12 myoblasts. The immunoglobulin G (IgG) was used as the control group. (c) Flag‐P4HB plasmid was transfected into C2C12 myoblast, and exogenous Flag‐tag was detected by the co‐immunoprecipitation assay with anti‐PHGDH antibody. (d) Immunofluorescence showed that endogenous PHGDH and P4HB colocalized in C2C12 myoblasts. Scale bars, 30 μm. (e) In vitro GST pull‐down assay to verify the interaction between P4HB and PHGDH. C2C12 myoblasts were transfected with GST alone or GST‐PHGDH. Retrieved proteins were evaluated with immunoblotting. GST‐immunocomplexes were boiled and subjected to immunoblotting analysis using P4HB antibody. (f) Left panel: A schematic diagram shows the structural domains of P4HB. Right panel: The control vector, Flag‐tagged wild type or truncated mutants of P4HB were transfected into C2C12 myoblasts. The cell lysates were subjected to the co‐immunoprecipitation assay by anti‐FLAG M2 Magnetic Beads and PHGDH expression was analyzed by immunoblotting. (g) P4HB plasmid was transfected into C2C12 myoblasts for 48 h and mRNA levels of P4HB and PHGDH were analyzed by RT‐qPCR assay (top panel). The protein levels of P4HB and PHGDH were analyzed by immunoblotting (bottom panel). (h) C2C12 myoblasts transfected with P4HB plasmid were treated with 100 μg/ml CHX and harvested at the indicated time point. The cell lysates were subjected to immunoblotting and PHGDH expression was quantified by ImageJ software. (i) HA‐Ub and V5‐PHGDH plasmid were co‐transfected with Flag‐P4HB plasmid in C2C12 myoblasts. After 36 h, cells were treated with 10 μM MG132 for 6 h. Anti‐V5 antibody was used to immunoprecipitate with exdogenous P4HB. Its ubiquitination level was assessed by anti‐HA antibody. 5% input of cell lysates was used to analyze the expression of P4HB and PHGDH
