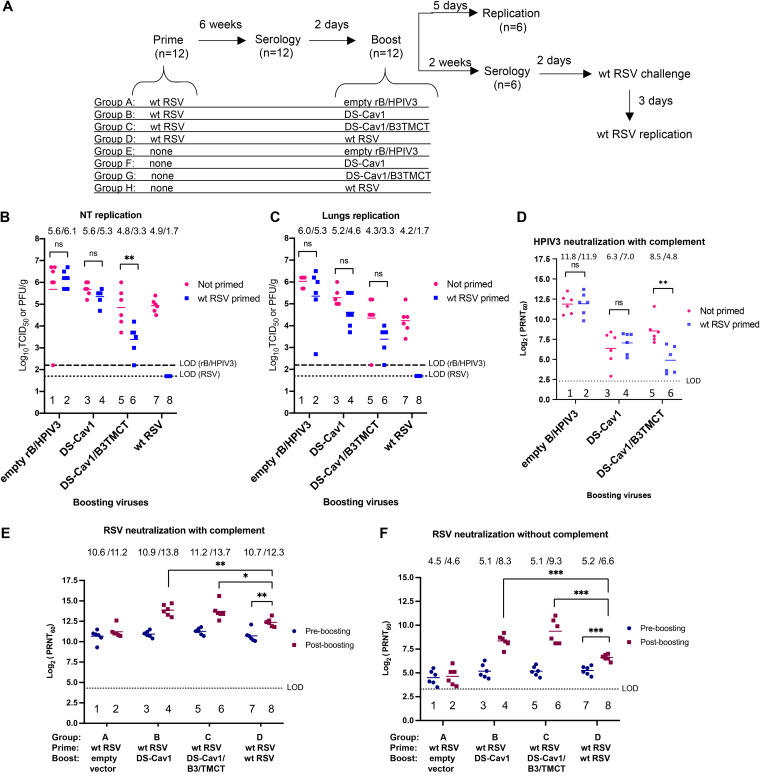
FIG 1.
Evaluation of a prime-boost regimen in hamsters. (A) Study design. Ninety-six female hamsters were confirmed to be RSV and HPIV3 seronegative and were assigned into eight groups of 12 animals each. On day 0, groups A to D were given a primary i.n. infection with 106 PFU of wt RSV in 0.1 ml of L15 medium, and groups E to H were left uninfected. Six weeks later, sera were collected for measurement of pre-boost 60% RSV-PRNTs. Two days later, primed and unprimed groups in pairs were boosted i.n. (105 TCID50 for vectors and 106 PFU for RSV) with one of the four viruses, i.e., (i) the empty rB/HPIV3 vector (groups A and E), (ii) DS-Cav1 vector (groups B and F), (iii) DS-Cav1/B3TMCT vector (groups C and G), or (iv) wt RSV (groups D and H). Five days after boosting, NTs and lungs of 6 hamsters per group were collected for virus titration. From the other 6 hamsters per group, sera were collected 2 weeks after boosting to measure RSV- and HPIV3-PRNTs; 2 days later, the animals were challenged i.n. with 106 PFU wt RSV. Three days following the challenge, NTs and lungs were collected for titration of RSV replication. (B and C) Titers of boosting viruses in NT (B) and lung (C) tissue homogenates 5 days post-boost. Dashed and dotted lines indicate the limit of detection (LOD) for the rB/HPIV3 vectors and wt RSV, respectively. (D) Serum HPIV3-PRNTs 2 weeks post-boost in primed and unprimed hamsters, assayed with complement. (E and F) Pre-boost and post-boost serum RSV-PRNTs in animals from groups A to D, which were primed with wt RSV and boosted with the indicated viruses, assayed with (E) and without (F) added complement. The significance of differences between the indicated comparisons was determined by Student's t test. ns, not significant (P > 0.05); *, 0.01 < P < 0.05; **, 0.001 < P < 0.01; ***, 0.0001 < P < 0.001.