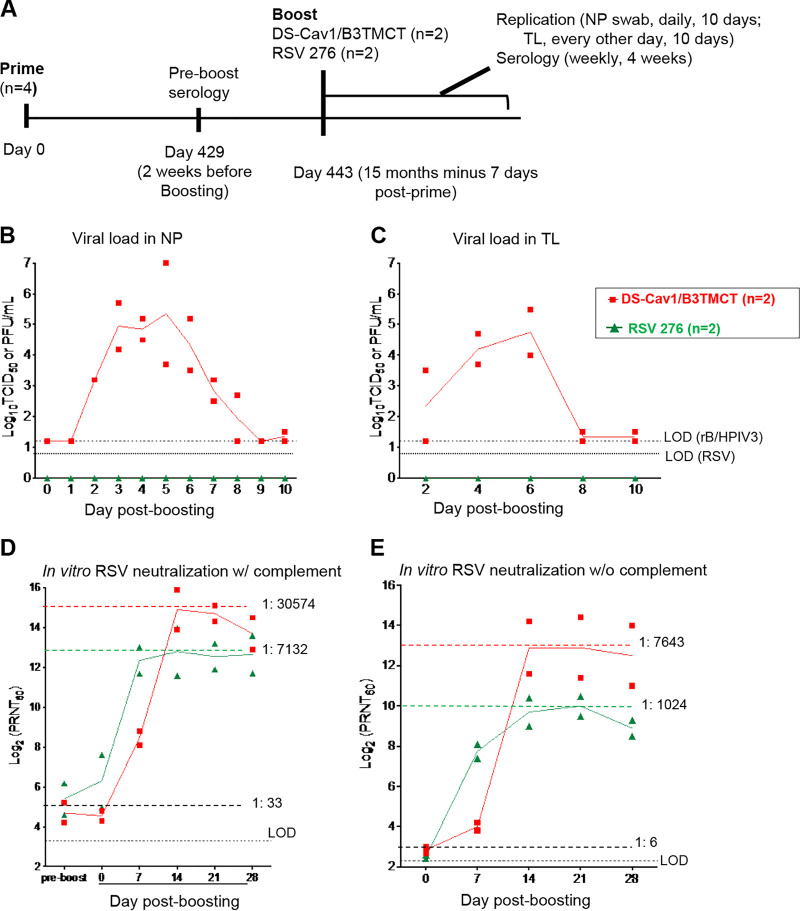
FIG 4.
AGM experiment 3: viral replication and serum RSV-PRNTs in AGMs when the interval between priming and boosting was ∼15 months (15 months minus 7 days). (A) Study design. Four AGMs were previously administered a primary infection with the live-attenuated vaccine candidate RSV 276 (Table 3). Sera were collected on day 429 (2 weeks before boosting) and RSV-PRNTs were measured with complement. The AGMs were distributed into two groups of 2 animals each, such that the groups had similar individual and mean RSV-PRNTs based on the day 429 sera (Table 3). On day 443 (15 months minus 7 days) postpriming, sera were collected and the two groups were boosted with DS-Cav1/B3TMCT vector (n = 2) or RSV 276 (n = 2) administered i.n. and i.t. at dose of 106 TCID50 (DS-Cav1/B3TMCT vector) or106 PFU (RSV 276) per site. Viral replication and serological responses were monitored as described in the legends to Fig. 2 and 3. (B and C) Viral titers in the NP (B) and TL (C) samples shown as means, with LODs shown as dashed lines. Symbols indicate titers for individual animals, and the lines indicates mean values. (D) Serum RSV-PRNTs at day 429 postpriming and days 0, 7, 14, 21, and 28 post-boosting, assayed with complement. (E) Serum RSV-PRNTs at days 0, 7, 14, 21, and 28 post-boosting, assayed without complement. Panels D and E are annotated to show the mean serum RSV-PRNTs for the combined two groups at the time of boosting (black dashed lines, with the mean arithmetic values shown); in addition, panels D and E are annotated with dashed colored lines that indicate the highest mean serum RSV-PRNT for each group, with the arithmetic values shown.