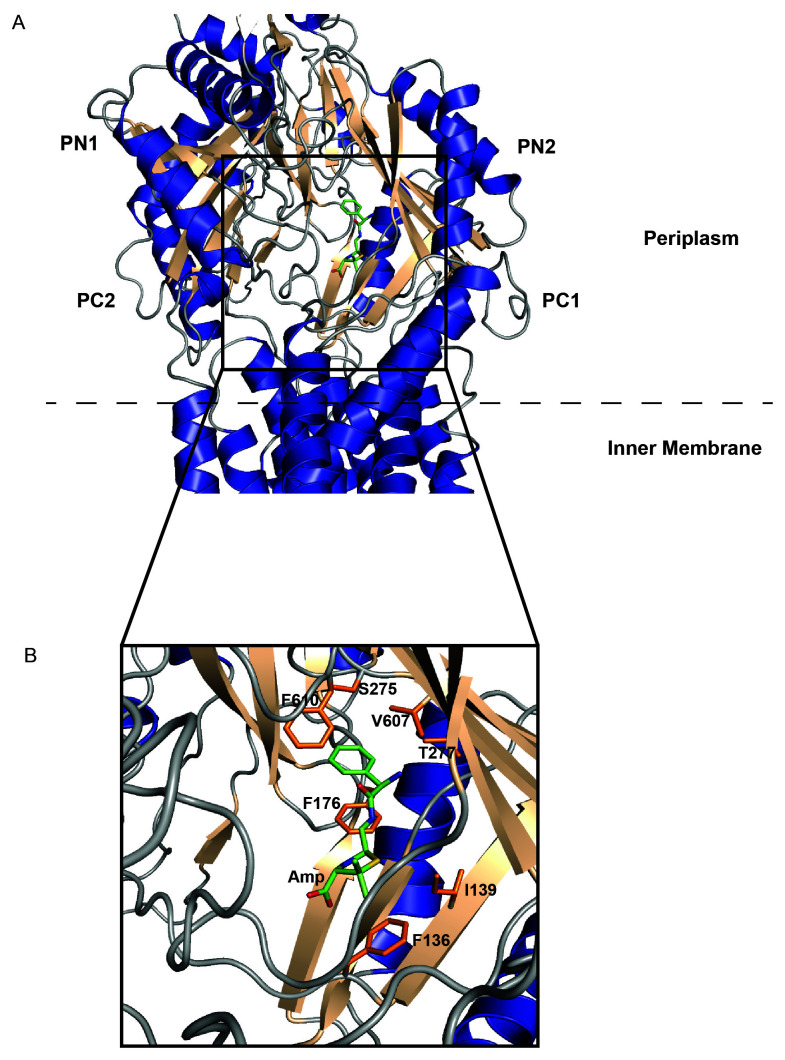
Figure 2. Antibiotic-bound cryo-EM structure of the Neisseria gonorrhoeae RND-type inner membrane pump, MtrD (adapted from Protein Data Bank ID 6VKS).
(A) α-helices (blue), β-sheets (wheat), and loops (gray) depict the overall secondary structure of MtrD. A hydrolyzed, decarboxylated ampicillin molecule (green) is bound deep within the cavity formed by the orientation of the periplasmic domains PC1, PC2, PN1, and PN2. The inner membrane–periplasm lipid boundary is represented by a dashed line. (B) A magnified view of the ampicillin-binding region. Important amino acid side chains involved in substrate recognition/stabilization are shown in orange. Amp, hydrolyzed, decarboxylated ampicillin; cryo-EM, cryo-electron microscopy; RND, resistance–nodulation–cell division.